La MOLE | Lezioni di Chimica
Summary
TLDRIn this lesson, the concept of the mole is introduced as a crucial unit of measurement in chemistry. The mole bridges the microscopic world of atoms and molecules with the macroscopic world we can measure. By using the mole and Avogadro's number (6.022 x 10^23), chemists can calculate precise amounts of substances for reactions, ensuring efficiency and accuracy. The lesson explains how to convert between grams, moles, and particles, and emphasizes the importance of understanding moles for solving chemistry problems. The video also touches on the significance of mass molar calculations and how to apply formulas to determine quantities in lab work.
Takeaways
- 😀 Atoms are too small and light to be weighed directly, so chemists need a way to work with large quantities of atoms or molecules.
- 😀 The mole is a unit of measurement used in chemistry to bridge the gap between the microscopic world of atoms and molecules and the macroscopic world of substances we can measure.
- 😀 The mole allows chemists to calculate how many atoms or molecules are in a substance by converting its mass into moles and linking it to quantities we can work with.
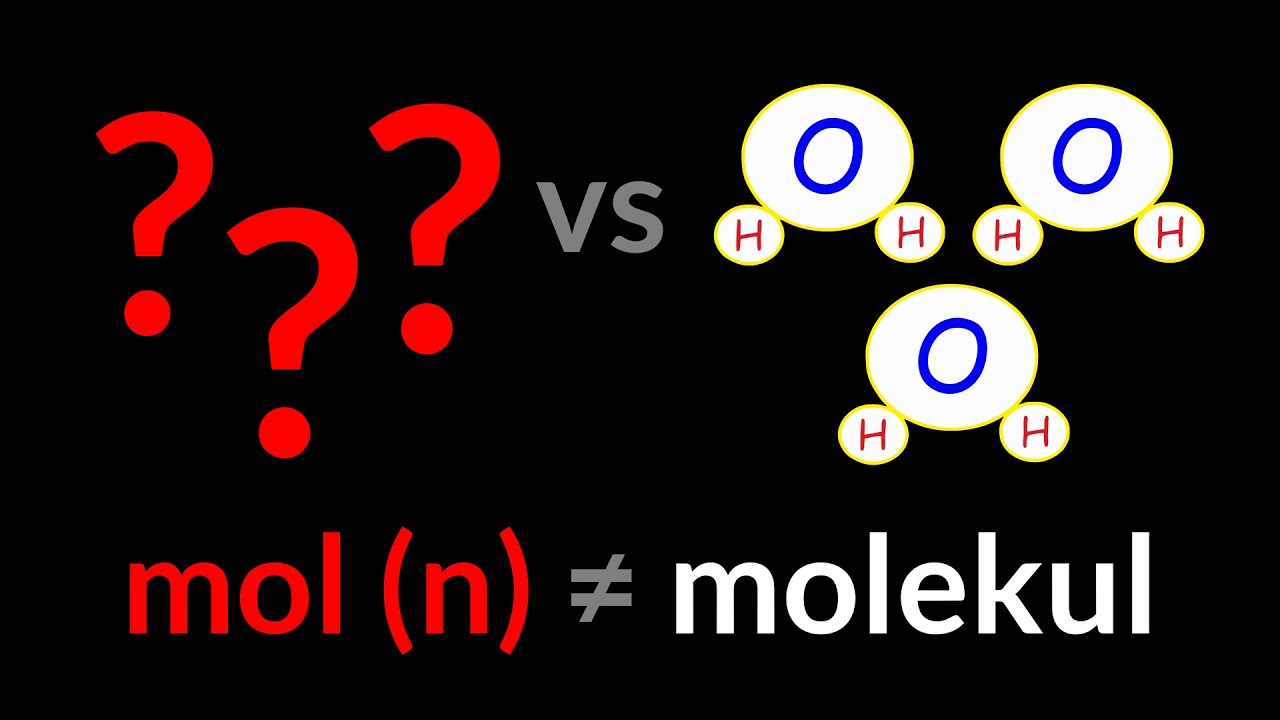
- 😀 To understand the mole, think of it as a counting unit like a dozen: 1 mole equals 6.022 × 10^23 particles, also known as Avogadro’s number.
- 😀 One mole of hydrogen atoms weighs 1 gram, and one mole of oxygen atoms weighs 16 grams, allowing scientists to calculate the number of atoms or molecules in a given mass.
- 😀 The definition of a mole is based on the number of particles (atoms, molecules) in 12 grams of carbon-12, which is 6.022 × 10^23 particles.
- 😀 Avogadro's number (6.022 × 10^23) represents the number of particles in one mole of a substance, whether it's atoms, molecules, or any other particles.
- 😀 The mass of one mole of a substance, known as the molar mass, is expressed in grams per mole (g/mol), and it corresponds to the molecular or atomic mass of the substance.
- 😀 The formula to convert mass to moles is: moles = mass (in grams) / molar mass (in g/mol). This formula helps chemists calculate the number of moles in a substance they’ve weighed.
- 😀 To find the number of particles in a substance, you multiply the number of moles by Avogadro’s number: particles = moles × 6.022 × 10^23.
- 😀 Understanding the mole and its relationships with mass and number of particles is essential for solving most chemistry problems, especially when dealing with chemical reactions and calculations.
Q & A
What is the Mole in chemistry?
-The Mole is a fundamental unit of measurement in chemistry used to quantify the amount of substance. It represents the quantity of particles (atoms, molecules, etc.) present in a specific amount of matter, typically defined as 6.022 × 10^23 particles.
Why is the Mole necessary in chemistry?
-The Mole is necessary because atoms are too small and light to be measured directly in the lab. By using the Mole, we can bridge the gap between the microscopic world of atoms and molecules and the macroscopic world that we can measure and observe, such as grams or liters.
What is Avogadro's number, and why is it important?
-Avogadro's number, 6.022 × 10^23, is the number of particles (atoms, molecules, etc.) in one mole of a substance. This number allows us to relate the atomic or molecular scale to macroscopic quantities like grams, making it essential for chemical calculations.
How does the Mole help in chemical reactions?
-The Mole helps in chemical reactions by enabling accurate calculations of reactants and products. By knowing the number of moles of each substance involved, chemists can predict how much of each substance will be used or produced in a reaction, minimizing waste and maximizing efficiency.
How is the Mole defined based on carbon-12?
-The Mole is defined as the amount of substance that contains the same number of particles as there are in 12 grams of carbon-12. This definition uses carbon-12 because it is abundant and widely present in organic compounds, making it a practical reference for measuring moles.
What is the difference between atomic mass and molar mass?
-Atomic mass is the mass of a single atom, typically expressed in atomic mass units (amu), while molar mass is the mass of one mole of a substance, usually expressed in grams per mole (g/mol). The molar mass corresponds to the atomic or molecular mass but in a practical, measurable quantity for bulk substances.
What does it mean when a substance has a molar mass of 18 g/mol?
-A molar mass of 18 g/mol means that one mole of that substance (such as water) weighs 18 grams. This mass corresponds to the total mass of 6.022 × 10^23 molecules of that substance.
How do you calculate the number of moles in a given mass of a substance?
-To calculate the number of moles in a substance, divide the mass of the sample (in grams) by the molar mass of the substance (in grams per mole). The formula is: moles = mass (g) / molar mass (g/mol).
How do you calculate the number of particles in a given number of moles?
-To calculate the number of particles in a given number of moles, multiply the number of moles by Avogadro's number (6.022 × 10^23). The formula is: number of particles = moles × Avogadro's number.
What is the relationship between moles, mass, and particles in a chemical reaction?
-In a chemical reaction, the number of moles of each substance is related to the mass of the substance and the number of particles it contains. By converting between moles, mass, and particles, chemists can determine how much of each substance is involved in a reaction and calculate the amounts of reactants and products needed.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführen5.0 / 5 (0 votes)