S9Q2W7 | THE MOLE
Summary
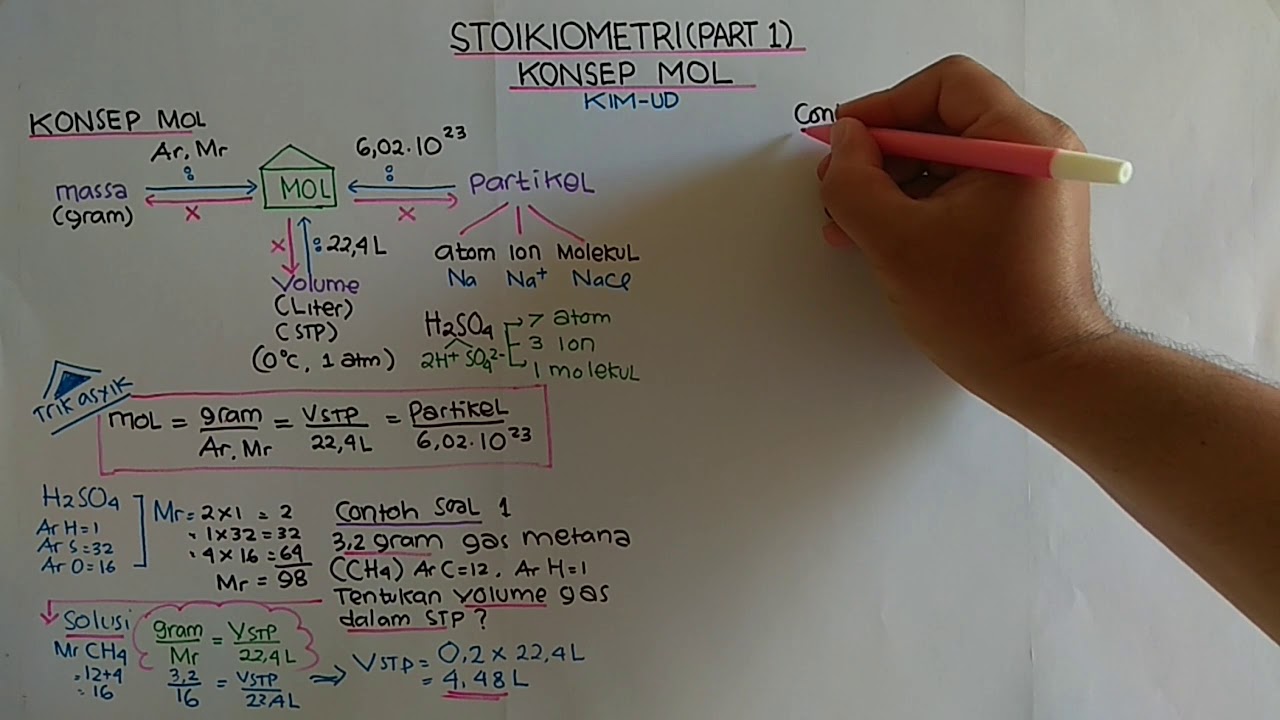
TLDRThis educational video lesson introduces the concept of the mole in chemistry, explaining its significance and how it is used to measure substances. The lesson covers Avogadro's number, molar mass, and how to calculate the number of molecules or atoms in a substance using moles. Through practical examples, such as calculating the moles of aluminum and methane, the video demonstrates how to apply the mole concept to solve real-world chemistry problems. By the end, viewers will understand how to use the mole for conversions and calculations in chemical reactions.
Takeaways
- 😀 The mole concept in chemistry is used to measure the amount of substance, just like how a dozen measures 12 items.
- 😀 Avogadro's number, 6.02 × 10^23, represents the number of particles in one mole of a substance.
- 😀 Mole is a fundamental unit in chemistry, defined as the number of atoms in exactly 12 grams of carbon-12.
- 😀 Avogadro's number is named after Italian physicist Amedeo Avogadro, whose hypothesis became a fundamental scientific law.
- 😀 1 mole of any substance equals 6.02 × 10^23 particles, whether they are atoms, molecules, or ions.
- 😀 To calculate molecules from moles, you multiply the number of moles by Avogadro's number.
- 😀 A mole's mass, also called molar mass, is the mass in grams of 1 mole of a substance, and it can be calculated from the periodic table.
- 😀 The molar mass of a compound is calculated by adding the atomic masses of the elements it contains, multiplied by their respective quantities.
- 😀 To convert from grams to moles, divide the given mass by the molar mass of the substance.
- 😀 The mole concept allows chemists to easily calculate the number of atoms or molecules in a given sample of any substance.
Q & A
What is the mole concept in chemistry?
-The mole is a fundamental unit in chemistry used to measure the amount of substance. One mole contains 6.02 × 10^23 particles of a substance, which is known as Avogadro's number.
Who is Avogadro, and what did he contribute to the concept of moles?
-Amedeo Avogadro was an Italian physicist who, in the 18th century, proposed that equal volumes of gases, at the same temperature and pressure, contain the same number of molecules. His work led to the establishment of Avogadro's number (6.02 × 10^23), which defines the number of particles in one mole of a substance.
How does Avogadro's number relate to the mole?
-Avogadro's number (6.02 × 10^23) represents the number of particles (atoms, molecules, ions, etc.) in one mole of any substance. It allows chemists to convert between moles and the number of particles.
What is the molar mass of a substance?
-The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole. It is numerically equal to the atomic or molecular mass of the substance, typically found on the periodic table.
How do you calculate the number of molecules in a given number of moles?
-To calculate the number of molecules in a given number of moles, multiply the number of moles by Avogadro's number (6.02 × 10^23). For example, 4.0 moles of carbon dioxide would contain 4.0 × 6.02 × 10^23 = 2.41 × 10^24 molecules.
What does the term 'mole' mean in chemistry?
-The term 'mole' comes from the Latin word 'mole', meaning a large heap or mass. In chemistry, it refers to the amount of a substance that contains 6.02 × 10^23 particles, providing a way to count particles at the atomic or molecular level.
How do you convert from grams to moles?
-To convert from grams to moles, divide the mass of the substance in grams by its molar mass. For example, to convert 10.0 grams of aluminum to moles, divide 10.0 by the molar mass of aluminum (26.98 g/mol), resulting in 0.371 moles.
What is the molar mass of methane (CH4)?
-The molar mass of methane (CH4) is calculated by adding the atomic masses of carbon and hydrogen. Carbon has an atomic mass of 12.01 g/mol, and hydrogen has an atomic mass of 1.008 g/mol. The total molar mass of CH4 is 12.01 + (4 × 1.008) = 16.04 g/mol.
How can you calculate the number of atoms in a given number of moles?
-To calculate the number of atoms in a given number of moles, multiply the number of moles by Avogadro's number. For example, 0.371 moles of aluminum would contain 0.371 × 6.02 × 10^23 = 2.23 × 10^23 atoms.
What is the process to calculate the molar mass of ferric oxide (Fe2O3)?
-To calculate the molar mass of ferric oxide (Fe2O3), find the atomic masses of iron (Fe) and oxygen (O) from the periodic table. Iron has an atomic mass of 55.85 g/mol, and oxygen has 16.0 g/mol. For Fe2O3, the molar mass is (2 × 55.85) + (3 × 16.0) = 159.7 g/mol.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)