Pharmacology - OPIOIDS (MADE EASY)
Summary
TLDRThis lecture delves into the pharmacology of opioids, explaining their mechanism of action in pain relief and euphoria through interaction with the central nervous system. It details the transmission of pain, the role of neurotransmitters like glutamate and substance P, and the body's endogenous opioids. The video also covers opioid receptors, synthetic agonists, and the side effects and addiction risks associated with opioid use. It concludes with a discussion on partial agonists like Buprenorphine and the antagonist Naloxone, crucial in reversing opioid overdoses.
Takeaways
- 💊 Opioids are drugs that act on the central nervous system to provide pain relief and euphoria, similar to morphine.
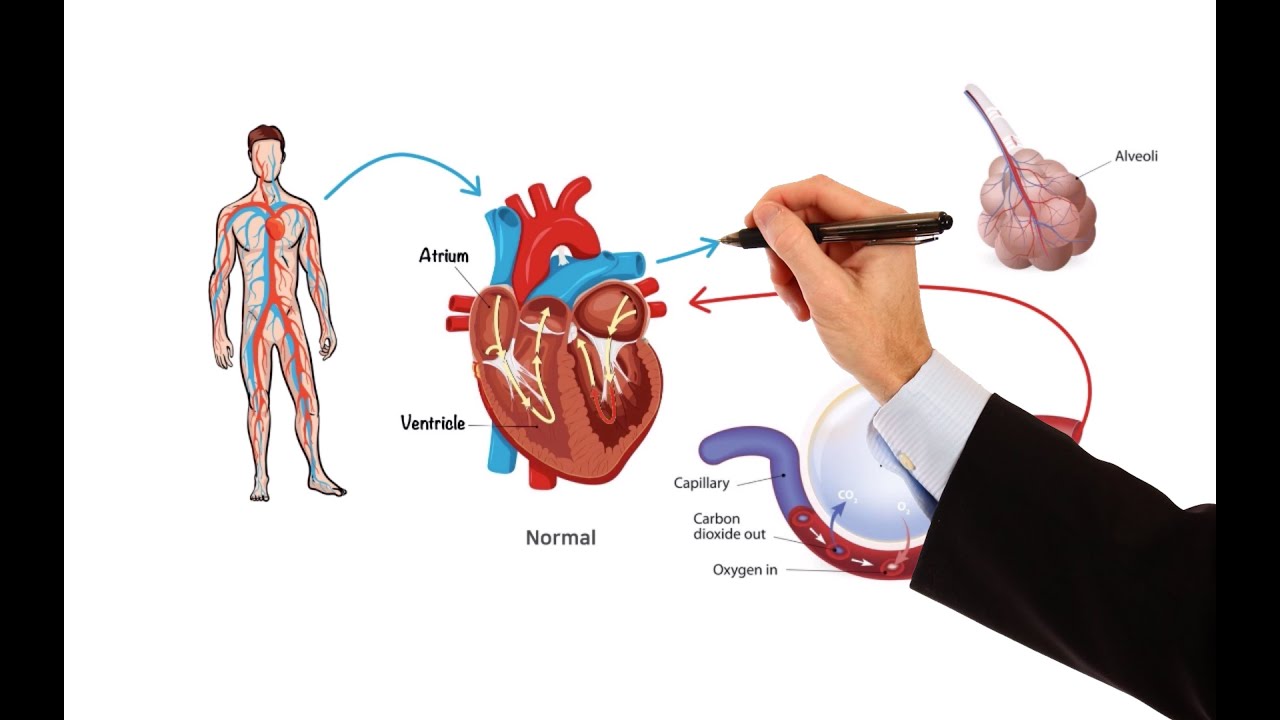
- 🧠 Pain transmission starts at nociceptors in the peripheral nervous system and is processed through the spinal cord to the brain.
- ⚡ Pain signals are carried as action potentials and involve neurotransmitters like glutamate, substance P, and CGRP.
- 📈 Glutamate excites neurons by activating NMDA and AMPA receptors, increasing calcium and sodium ion influx.
- 🔄 Endogenous opioids (enkephalins, dynorphins, and endorphins) bind to opioid receptors (μ, δ, k) to reduce pain by inhibiting neurotransmitter release.
- 🚀 Synthetic opioids, such as Fentanyl and Oxycodone, are more potent than naturally-derived opioids.
- 🤒 Opioids can cause side effects like nausea, respiratory depression, immune suppression, and constipation.
- 🔄 Opioid addiction involves GABA-inhibitory interneurons and increased dopamine release, leading to euphoria.
- 🚨 Naloxone is an opioid antagonist used to reverse the effects of opioid overdose by blocking receptors.
- 🧬 Prolonged opioid use leads to tolerance and withdrawal symptoms, which are opposite to the drugs' effects.
Q & A
What are opioids and how do they affect the central nervous system?
-Opioids, sometimes called narcotics, are a group of drugs that act on the central nervous system to produce morphine-like effects such as pain relief and euphoria.
What is the primary function of nociceptors in pain transmission?
-Nociceptors are the branching ends of sensory neurons in the peripheral nervous system that respond to body damage by transmitting the painful stimulus to second-order neurons in the dorsal horn of the spinal cord.
How does glutamate contribute to the transmission of pain signals?
-Glutamate activates both NMDA and AMPA receptors, permitting the influx of positively charged calcium and sodium ions, which makes neurons more likely to fire, thereby exciting second-order neurons in the dorsal horn and propagating a sharp, localized pain signal.
What role does Substance P play in pain transmission?
-Substance P binds to NK-1 receptors, leading to intracellular signaling that activates arachidonic acid pathways, nitric oxide synthesis, and NMDA receptors. This increases the pain signal and makes neurons more likely to fire.
What are the three major families of endogenous opioids, and how do they function?
-The three major families of endogenous opioids are enkephalins, dynorphins, and endorphins. They exert their effects by binding to opioid receptors, which are present in the central and peripheral nervous systems, thereby inhibiting pain transmission.
How do opioid receptors inhibit the transmission of pain?
-Activation of opioid receptors by an agonist causes the closing of voltage-gated calcium channels on presynaptic nerve terminals, decreasing neurotransmitter release, and opening potassium channels, resulting in hyperpolarization and reduced neuron sensitivity to excitatory inputs.
What are some examples of synthetic opioid agonists, and how do they differ from naturally derived opioids?
-Examples of synthetic opioid agonists include Fentanyl, Hydrocodone, Hydromorphone, Methadone, Meperidine, Oxycodone, and Oxymorphone. They are more potent than naturally derived opioids due to refined processing.
What are the common side effects associated with opioid use?
-Common side effects include nausea, respiratory depression, antitussive effect, suppression of the immune system, hypotension, dilation of cutaneous blood vessels, tachycardia or bradycardia, itching, constipation, renal function depression, and urinary retention.
How does prolonged use of opioids lead to addiction and withdrawal symptoms?
-Prolonged opioid use leads to desensitization and down-regulation of receptors, decreasing sensitivity to opioids. When use is reduced or stopped, withdrawal symptoms manifest as opposite effects to those of the opioids, such as diarrhea, elevated blood pressure, dysphoria, and anxiety.
What is Naloxone and how is it used in opioid overdose situations?
-Naloxone is an opioid antagonist that blocks or reverses the effects of opioids by knocking off the opioids attached to brain receptors, restoring normal breathing during an overdose.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen
5.0 / 5 (0 votes)