TERMOKIMIA part 2- jenis-jenis perubahan entalpi standar Kimia kelas 11 semester 1
Summary
TLDRThis video from the Smart Chemistry channel continues the discussion on thermochemistry, focusing on standard enthalpy changes under standard conditions (25°C and 1 atm). It explains the concept of standard enthalpy change (ΔH⁰) and introduces three main types: formation, decomposition, and combustion, detailing their definitions, symbols, and key characteristics. The video provides clear examples, such as the formation of H₂O, decomposition of NH₃, and combustion of methane and acetylene, including energy values and proper thermochemical equations. Step-by-step problem-solving demonstrates how to write thermochemical equations, making it easier for students to identify reaction types and understand energy changes in chemical reactions.
Takeaways
- 😀 Standard enthalpy change (ΔH°) is measured at 25°C and 1 atm, representing standard conditions.
- 😀 ΔH° is influenced by two main factors: temperature and pressure.
- 😀 The symbol for standard enthalpy change includes a superscript zero (ΔH°) to indicate standard conditions.
- 😀 There are three main types of standard enthalpy changes for Grade 11 students: formation, decomposition, and combustion.
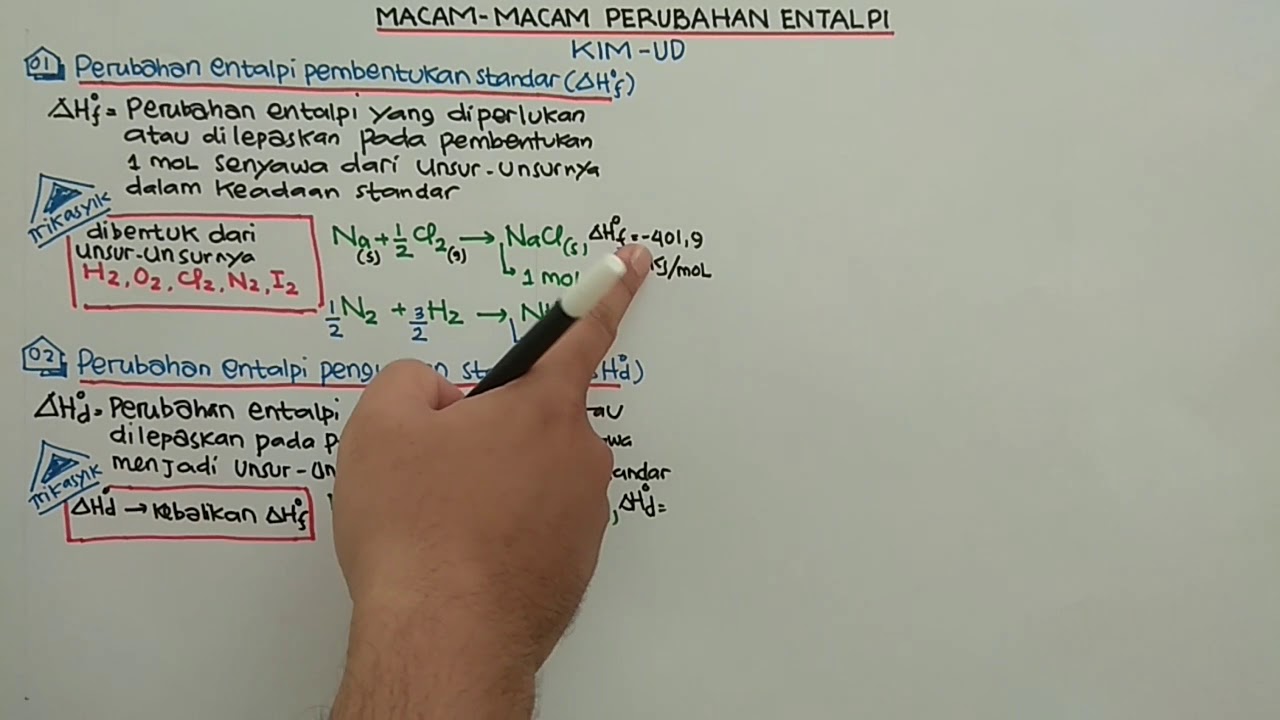
- 😀 Standard enthalpy of formation (ΔHf°) is the enthalpy change for forming 1 mole of a compound from its elements under standard conditions.
- 😀 The formation reaction example: H₂ + ½ O₂ → H₂O, with ΔHf° = −285.85 kJ/mol.
- 😀 Standard enthalpy of decomposition (ΔHd°) is the enthalpy change for decomposing 1 mole of a compound into its elements, and its value is the opposite of ΔHf°.
- 😀 Standard enthalpy of combustion (ΔHc°) is the enthalpy change for burning 1 mole of a substance in oxygen under standard conditions.
- 😀 Exothermic reactions release energy (ΔH negative), while endothermic reactions require energy (ΔH positive).
- 😀 Thermochemical equations must be complete: balanced, include ΔH with the correct sign, indicate standard states, and have the compound coefficient as 1 mole.
- 😀 Example exercises from the video illustrate formation, decomposition, and combustion reactions with their respective ΔH values and proper balancing.
- 😀 Symbols: ΔHf° for formation, ΔHd° for decomposition, and ΔHc° for combustion help categorize reactions and understand energy changes.
Q & A
What is the definition of standard enthalpy change?
-The standard enthalpy change (ΔH⁰) is the enthalpy change for a chemical reaction measured under standard conditions, which are 25°C temperature and 1 atm pressure.
What factors influence enthalpy change (ΔH)?
-Enthalpy change is influenced by temperature and pressure.
What is the symbol for standard enthalpy change and why is it written that way?
-The symbol for standard enthalpy change is ΔH⁰. The superscript '0' indicates that the measurement is taken under standard conditions.
What is the standard enthalpy of formation?
-The standard enthalpy of formation (ΔHf⁰) is the enthalpy change for forming one mole of a compound directly from its elements under standard conditions.
Provide an example of a standard enthalpy of formation reaction.
-The formation of water: H₂(g) + ½ O₂(g) → H₂O(l); ΔHf⁰ = −285.85 kJ/mol.
What is the standard enthalpy of decomposition?
-The standard enthalpy of decomposition (ΔHd⁰) is the enthalpy change for decomposing one mole of a compound into its constituent elements under standard conditions.
How is the enthalpy of decomposition related to the enthalpy of formation?
-The enthalpy of decomposition has the same magnitude as the enthalpy of formation but with the opposite sign.
What is the standard enthalpy of combustion?
-The standard enthalpy of combustion (ΔHc⁰) is the enthalpy change when one mole of a substance is completely burned in oxygen under standard conditions.
Give an example of a thermochemical equation for combustion.
-Combustion of methane: CH₄(g) + 2 O₂(g) → CO₂(g) + 2 H₂O(g); ΔHc⁰ = −393.5 kJ/mol.
What key information must a thermochemical equation include?
-A thermochemical equation must include the reaction, the phases of reactants and products, and the enthalpy change (ΔH).
How do you determine the sign of ΔH in a thermochemical equation?
-The sign of ΔH is negative (−) for exothermic reactions, which release energy, and positive (+) for endothermic reactions, which absorb energy.
In a formation reaction, why is it important that the substance formed is one mole?
-The ΔHf⁰ value corresponds to the formation of exactly one mole of the compound, so the coefficient must be one to accurately represent standard enthalpy of formation.
What is the thermochemical equation for the decomposition of NH₃ under standard conditions requiring 46 kJ/mol?
-2 NH₃(g) → N₂(g) + 3 H₂(g); ΔHd⁰ = +46 kJ/mol.
Why must combustion reactions always include O₂?
-Because combustion is defined as a reaction in which a substance reacts completely with oxygen to release energy, forming CO₂ and H₂O in the case of hydrocarbons.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen

Animasi Entalpi Molar - Termokimia Part 5 🔥🌡

Termokimia (3) | Jenis - Jenis Perubahan Entalpi Standar

Hukum Termodinamika, Bagian 3: Termokimia

PENENTUAN PERUBAHAN ENTALPI REAKSI BERDASARKAN ENTALPI PEMBENTUKAN STANDAR
MACAM - MACAM PERUBAHAN ENTALPI

PERUBAHAN ENTALPI STANDAR ( BAB TERMOKIMA - MAPEL KIMIA KELAS 11 )
5.0 / 5 (0 votes)
