O Átomo de Bohr Explicado
Summary
TLDRThis video explores the evolution of atomic models, starting with Thomson's atomic model and moving to Rutherford’s planetary model. While Rutherford’s model addressed some problems, it introduced new issues, especially regarding atomic stability. The video explains the limitations of Rutherford’s model in light of atomic spectra and introduces Bohr's model, which incorporates quantum mechanics. Bohr's theory resolved many of the stability issues and accurately explained atomic spectra, marking a significant shift from classical physics. The video ends by reflecting on the course’s impact and the importance of accessible education.
Takeaways
- 😀 Rutherford's atomic model solved some issues in Thomson's model but introduced new problems, particularly regarding atomic stability.
- 😀 A planetary model of the atom, with electrons orbiting the nucleus, is unstable due to electromagnetic radiation emission from accelerating electrons.
- 😀 The emission of radiation by electrons in motion leads to a loss of energy, causing them to spiral inward and eventually collide with the nucleus.
- 😀 The classical theory failed to explain atomic light spectra, which led to the need for a new approach to understanding atomic behavior.
- 😀 Spectroscopy, the study of light emitted by atoms, revealed that atoms emit and absorb light at specific wavelengths, a phenomenon not explained by classical physics.
- 😀 The discovery of specific emission and absorption spectra by atoms helped identify elements, including the identification of helium in the sun before Earth.
- 😀 The Bohr model of the atom introduced the concept of quantized energy levels, resolving the instability issue of Rutherford's model by limiting electron orbits.
- 😀 Bohr's model proposed that electrons could only occupy specific orbits with discrete energy levels, preventing them from continuously emitting radiation.
- 😀 When an electron transitions between orbits, it emits light corresponding to the difference in energy between the two levels, forming discrete spectral lines.
- 😀 The Bohr model was groundbreaking because it successfully explained the spectral lines observed in experiments but still had limitations, leading to the development of quantum mechanics for a more complete understanding of atomic behavior.
Q & A
What problem did Rutherford's model fail to address in the atom's stability?
-Rutherford's model depicted the atom as a planetary system with electrons orbiting a central nucleus. However, it could not explain the stability of the atom. According to classical electromagnetic theory, orbiting electrons should emit radiation, lose energy, and spiral into the nucleus, causing the atom to collapse, which contradicts the observed stability of atoms.
How did Rutherford's model relate to the solar system, and why was this analogy problematic?
-Rutherford's model compared the atom to a solar system, with electrons orbiting a nucleus (the Sun). While the solar system is stable due to gravitational forces, the atomic model was problematic because the electrons, being charged, would accelerate and emit radiation according to classical electromagnetic theory, causing them to spiral inward and destabilize the atom.
What is the key insight of Bohr's model that resolved the instability issue of Rutherford's model?
-Bohr's model introduced the concept of quantized orbits. He proposed that electrons could only occupy specific, discrete orbits around the nucleus where their angular momentum is quantized. This eliminated the possibility of continuous radiation emission, thus preventing the electrons from spiraling into the nucleus.
What is the significance of the quantization of angular momentum in Bohr's model?
-The quantization of angular momentum is crucial because it restricts electrons to specific orbits with quantized energies. This prevents the electrons from emitting radiation continuously and losing energy. It also explains why only certain wavelengths of light are emitted when electrons transition between these orbits.
How does Bohr’s model explain atomic spectra?
-Bohr’s model explains atomic spectra through electron transitions between quantized energy levels. When an electron moves from a higher-energy orbit to a lower-energy orbit, it emits light with a specific frequency. These frequencies correspond to distinct lines in the atomic spectrum, which were experimentally observed and match the predictions made by Bohr.
What experimental evidence did Bohr’s model explain that Rutherford’s model could not?
-Bohr’s model explained the observed atomic spectra, which Rutherford’s model could not. According to Rutherford’s model, electrons should emit continuous radiation, but the actual spectra showed discrete lines, which Bohr accounted for by proposing that electrons can only exist in specific orbits, emitting light only during transitions between these orbits.
What role did the work of Newton and other scientists in the 17th century play in understanding atomic structure?
-Newton’s work on the prism in 1666 demonstrated that light can be separated into different colors, a discovery that led to the field of spectroscopy. This allowed scientists to analyze the light emitted by atoms and provided evidence for the quantized nature of atomic energy levels, laying the groundwork for later developments in atomic theory.
What are the main shortcomings of Bohr’s atomic model?
-Despite its success in explaining atomic spectra, Bohr’s model had limitations. It could not explain more complex atoms, especially those with multiple electrons, and it failed to account for fine details in spectral lines. Additionally, the mixing of classical and quantum theories in Bohr's model left physicists seeking a more complete quantum mechanical description.
Why was the quantization of energy levels necessary for the stability of atoms in Bohr's model?
-The quantization of energy levels in Bohr’s model was necessary to explain the stability of atoms. Without quantization, electrons would continuously lose energy and spiral into the nucleus, causing the atom to collapse. By restricting electrons to specific orbits with discrete energy levels, Bohr ensured that electrons would not emit radiation in a continuous manner, thus maintaining atomic stability.
How did Bohr's model contribute to the development of quantum mechanics?
-Bohr’s model marked a crucial step toward the development of quantum mechanics. It introduced the idea that atomic energy levels are quantized, a concept that would be expanded upon with the development of quantum theory. Bohr’s work set the stage for future advances in quantum mechanics, even though his model itself was incomplete.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen

Química - Natureza elétrica da matéria e núcleo atômico (prof. Luiz Landim)

MODELOS ATÔMICOS: Dalton, Thomson, Rutherford e Rutherford-Bohr

MODELOS ATÔMICOS: Dalton, Thomson e Rutherford | QUER QUE DESENHE?

Resumo Modelos Atômicos | Resumindo
Chemistry & Physics: History of the Atom (Dalton, Thomson, Rutherford, and Bohr Models)
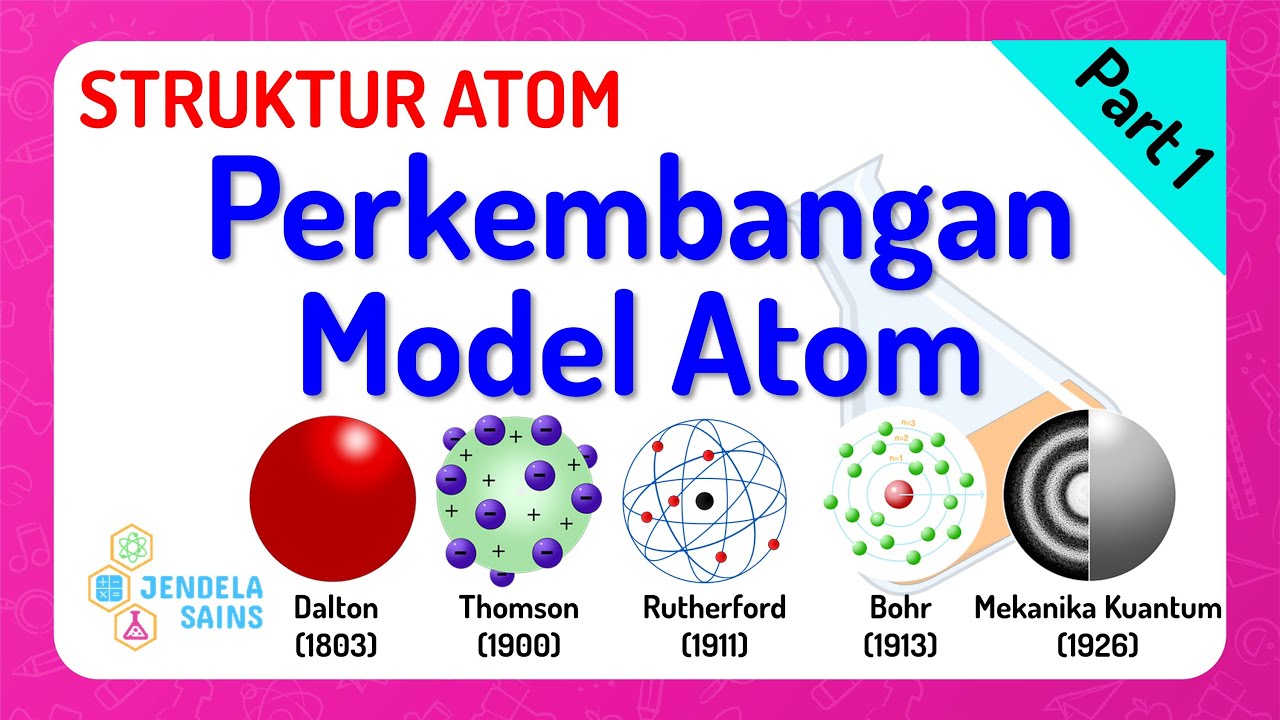
Struktur Atom • Part 1: Perkembangan Model Atom
5.0 / 5 (0 votes)
