Tests for Alcohols - MeitY OLabs
Summary
TLDRThis video explains various tests to identify alcohols, which are organic compounds containing the hydroxyl (-OH) group. It covers tests like the Sodium Metal test, Ester test, Ceric Ammonium Nitrate test, Acetyl Chloride test, Iodoform test, and Lucas test. Each test is detailed with materials and procedures, demonstrating how alcohols react with different chemicals. The reactions reveal distinctive properties, such as effervescence with sodium, fruity smells with acids, color changes with ceric ammonium nitrate, and the formation of iodoform or alkyl chlorides. These tests are essential for distinguishing between primary, secondary, and tertiary alcohols.
Takeaways
- 😀 Alcohols are organic compounds containing the hydroxyl functional group (-OH), formed by replacing a hydrogen atom in a hydrocarbon.
- 😀 Alcohols are classified into monohydric, dihydric, and trihydric based on the number of hydroxyl groups, and further into primary, secondary, and tertiary based on the carbon attached to the -OH group.
- 😀 Simple tests can be used to identify alcohols, including Sodium Metal Test, Ester Test, Ceric Ammonium Nitrate Test, Acetyl Chloride Test, Iodoform Test, and Lucas Test.
- 😀 In the Sodium Metal Test, alcohol reacts with sodium metal to produce effervescence due to the liberation of hydrogen gas.
- 😀 The Ester Test involves heating alcohol with glacial acetic acid and concentrated sulfuric acid to form an ester, which can be recognized by its fruity smell.
- 😀 In the Ceric Ammonium Nitrate Test, alcohol reacts with ceric ammonium nitrate to give a red color, indicating the formation of an alkoxy cerium (IV) complex.
- 😀 The Acetyl Chloride Test involves adding acetyl chloride to alcohol, which reacts to release hydrogen chloride gas. Ammonium hydroxide is then used to form white fumes of ammonium chloride.
- 😀 In the Iodoform Test, alcohol with a CH3-CH-OH group reacts with iodine and sodium hydroxide to form a yellow precipitate of iodoform (CHI3).
- 😀 The Lucas Test helps differentiate alcohol types: tertiary alcohol reacts immediately with Lucas reagent, secondary alcohol takes 1-5 minutes, and primary alcohol reacts only on heating.
- 😀 Each test relies on the reactivity of alcohols with specific reagents, enabling the identification of their structure and type.
Q & A
What are alcohols, and how are they classified?
-Alcohols are organic compounds containing the hydroxyl functional group (-OH). They are classified based on the number of hydroxyl groups as monohydric, dihydric, and trihydric. They are further classified as primary (1°), secondary (2°), and tertiary (3°) alcohols, depending on the position of the hydroxyl group attached to the carbon atom.
What is the purpose of the Sodium Metal Test for alcohols?
-The Sodium Metal Test helps identify alcohols by reacting with sodium metal, which releases hydrogen gas and produces effervescence when alcohols are present. This reaction occurs because alcohols, being active hydrogen donors, react with sodium.
What materials are required for the Ester Test, and what does it detect?
-The Ester Test requires an organic compound, glacial acetic acid, concentrated sulfuric acid, cold water, a test tube, droppers, and a water bath. This test detects the formation of esters when alcohols react with carboxylic acids in the presence of concentrated sulfuric acid, producing a fruity-smelling compound.
What color change occurs in the Ceric Ammonium Nitrate Test, and what does it indicate?
-In the Ceric Ammonium Nitrate Test, alcohols react with ceric ammonium nitrate to produce a red coloration. This indicates the formation of an alkoxy cerium (IV) complex, confirming the presence of an alcohol.
How does the Acetyl Chloride Test identify alcohols?
-In the Acetyl Chloride Test, alcohol reacts with acetyl chloride to release hydrogen chloride gas. The presence of white fumes of ammonium chloride, formed when the gas reacts with ammonium hydroxide, confirms the presence of alcohol.
What does the Iodoform Test detect in alcohols?
-The Iodoform Test detects alcohols containing the CH3-CH-OH group. When warmed with iodine solution and sodium hydroxide, these alcohols produce a yellow precipitate of iodoform (CHI3), confirming their presence.
What is the procedure for the Lucas Test, and what does it reveal about alcohols?
-In the Lucas Test, different alcohols (primary, secondary, and tertiary) are mixed with Lucas reagent. Tertiary alcohols react immediately, forming an alkyl chloride and causing cloudiness in the solution. Secondary alcohols take about 1-5 minutes to react, while primary alcohols require warming and take longer to react.
How does the Sodium Metal Test differ from the Ester Test?
-The Sodium Metal Test identifies alcohols by detecting effervescence due to hydrogen gas release when alcohol reacts with sodium. In contrast, the Ester Test identifies alcohols by forming a fruity-smelling ester when alcohol reacts with a carboxylic acid in the presence of sulfuric acid.
What is the role of anhydrous calcium sulfate in some alcohol identification tests?
-Anhydrous calcium sulfate is used in some alcohol tests, such as the Sodium Metal Test and Acetyl Chloride Test, to remove any water content from the organic compound before performing the reactions. This ensures accurate results by preventing interference from water.
Why is warming necessary in some alcohol tests like the Iodoform and Lucas Tests?
-Warming is necessary in tests like the Iodoform and Lucas Tests to facilitate the reactions between alcohols and reagents. In the Iodoform Test, warming speeds up the formation of the yellow precipitate, while in the Lucas Test, it helps primary alcohols react with Lucas reagent to form alkyl chlorides.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen

CBSE Class 12 || Chemistry || Alcohols, Phenols & Ethers || Part-I || Animation || in English
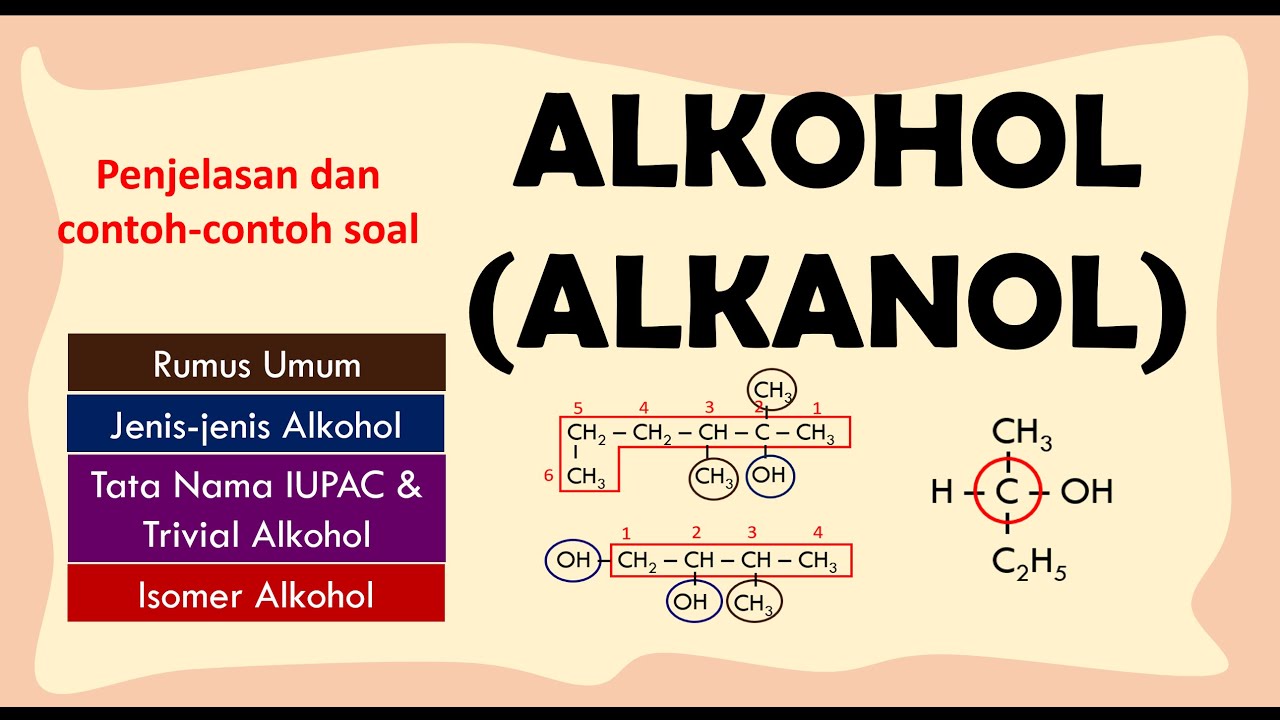
ALKOHOL (ALKANOL) : RUMUS UMUM, JENIS-JENIS ALKANOL, TATA NAMA IUPAC & TRIVIAL, ISOMER
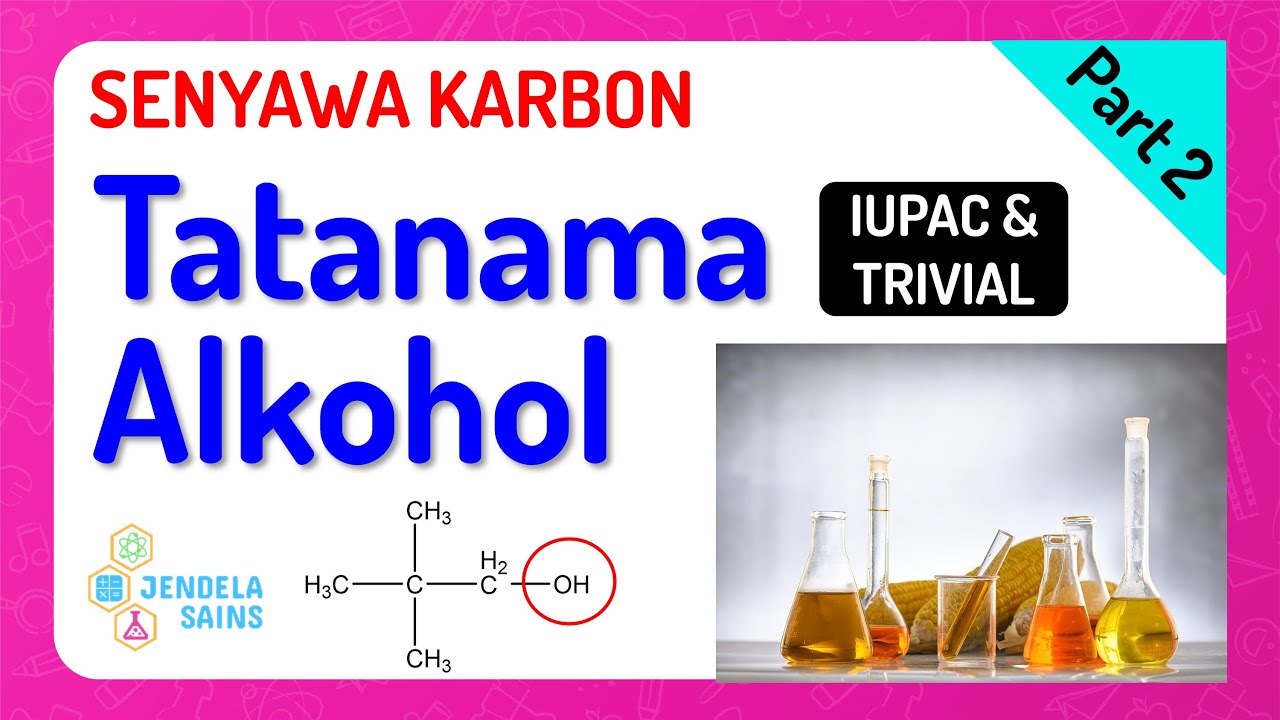
Senyawa Karbon Turunan Alkana • Part 2: Tatanama Alkohol / Alkanol

Qualitative Tests for Identification of Functional Groups

FUNÇÃO ÁLCOOL: QUÍMICA ORGÂNICA | Resumo para o Enem

Qualitative tests for organic functional groups – practical video | 16–18 years
5.0 / 5 (0 votes)
