FUNÇÃO ÁLCOOL: QUÍMICA ORGÂNICA | Resumo para o Enem
Summary
TLDRIn this video, the speaker introduces the alcohol functional group in organic chemistry, explaining its structure and uses. The alcohol function consists of a hydroxyl group attached to a saturated carbon, and the speaker uses ethanol as an example to illustrate its role as a fuel and in alcoholic beverages. The video also covers the basics of naming alcohols using a systematic approach based on the number of carbons and the position of the functional group. The importance of correct numbering and nomenclature for alcohol compounds is emphasized for students learning organic chemistry.
Takeaways
- 😀 Alcohol function contains a hydroxyl group (-OH) attached to a saturated carbon (a carbon without a double bond).
- 😀 Ethanol (C2H5OH) is the most well-known alcohol and is used as a fuel and in alcoholic beverages.
- 😀 Alcohols are commonly found in cleaning materials and as a component in fuels, such as gasoline additives.
- 😀 Alcohols are named based on the number of carbon atoms in the main chain and the position of the hydroxyl group.
- 😀 The prefix of alcohols is derived from the number of carbons, with 'pente' representing five carbons (pentanol).
- 😀 The hydroxyl group can appear at different positions in the carbon chain, and the smallest number is used when numbering the carbons.
- 😀 If the hydroxyl group is at the second position of a five-carbon chain, the molecule is named pentan-2-ol.
- 😀 Complex molecules may contain additional groups (e.g., methyl groups), and the position of these groups is also considered in naming.
- 😀 Alcohol nomenclature involves understanding the location of functional groups and radicals, especially with unsaturations or double bonds.
- 😀 The video encourages viewers to engage with the content by subscribing, liking, commenting, and completing related exercises for better understanding.
Q & A
What is the alcohol function in organic chemistry?
-The alcohol function consists of a hydroxyl group (-OH) attached to a saturated carbon atom. A saturated carbon is one that does not have any double bonds between carbon atoms.
What is the structure of ethanol?
-Ethanol has the molecular formula CH3CH2OH, consisting of two carbon atoms, five hydrogen atoms, and one hydroxyl group attached to the second carbon.
What are some common uses of alcohol?
-Alcohol, specifically ethanol, is used as a fuel, a component in alcoholic beverages, in cleaning products, and as an additive in gasoline.
How do you determine the nomenclature of an alcohol?
-The nomenclature of alcohols is determined by counting the carbon atoms in the main chain, identifying the position of the hydroxyl group, and applying the appropriate suffix '-ol' at the end of the name.
What is the prefix for a five-carbon chain in organic chemistry?
-The prefix for a five-carbon chain is 'pent-' (from the Greek word for five).
Why is the numbering of carbon atoms in the alcohol molecule important?
-Numbering the carbon atoms is important to correctly identify the position of the hydroxyl group and ensure the molecule's name reflects the functional group’s location on the chain.
What does 'pentanol' refer to?
-Pentanol refers to an alcohol molecule with a five-carbon chain and a hydroxyl group attached to one of the carbons in the chain. It can be named based on the position of the hydroxyl group, such as pentan-1-ol or pentan-2-ol.
What is the role of unsaturation in alcohol nomenclature?
-Unsaturation refers to the presence of double or triple bonds between carbon atoms. In alcohol nomenclature, the presence of unsaturation affects the numbering and suffix, as it needs to be considered in addition to the hydroxyl group.
What does the term 'methyl' refer to in the context of alcohol nomenclature?
-The term 'methyl' refers to a -CH3 group that can be attached to the main carbon chain in alcohols. It is used to name and number branches or radicals in more complex alcohol molecules.
How do you name a molecule with both a functional group and a methyl group?
-The molecule is named by first identifying the functional group and its position, then counting the carbon atoms in the main chain. The methyl group is added as a prefix with its position on the chain. For example, 3-methylpentan-1-ol indicates a methyl group at the third carbon of a pentanol chain.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Alcohols | Alcohols, ethers, epoxides, sulfides | Organic chemistry | Khan Academy
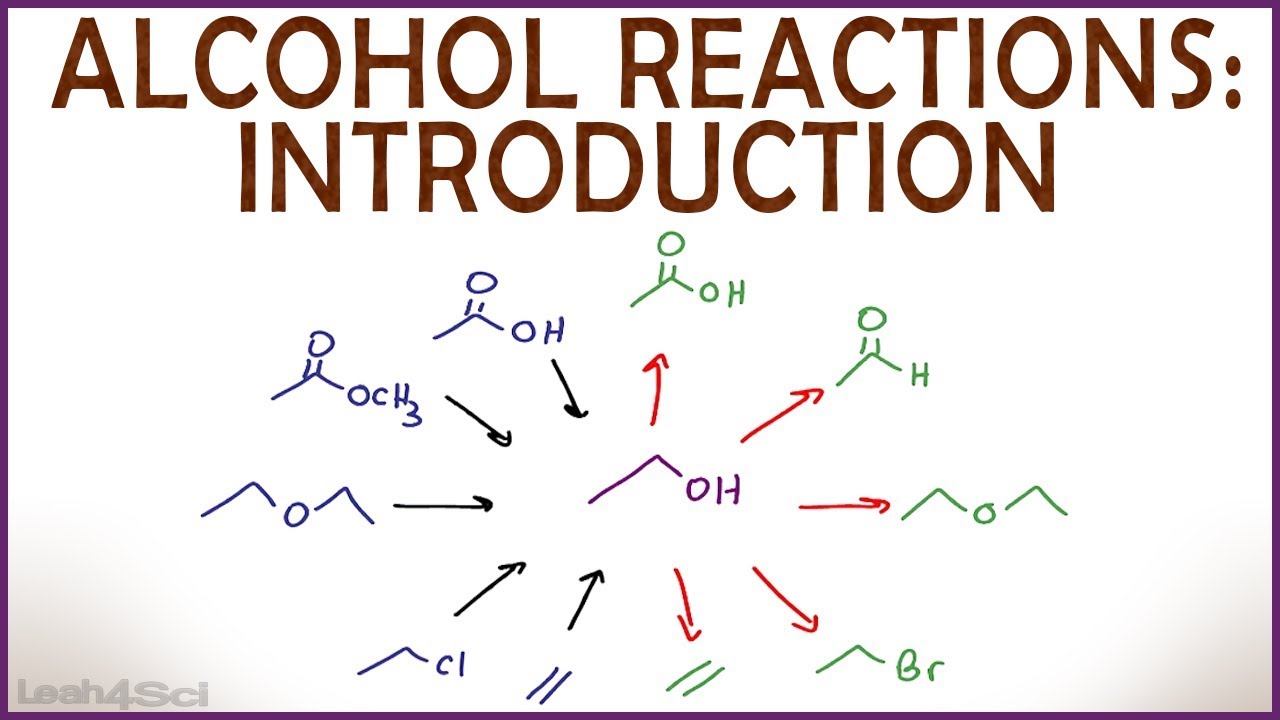
Introduction to Alcohol Properties and Reactions

MENGENAL GUGUS FUNGSI SENYAWA KARBON

Functional groups | Properties of carbon | Biology | Khan Academy

Kuliah Online Kimia PPKU - Pengenalan Kimia Organik Bagian 1 - Karakteristik Karbon dan Gugus Fungsi

BENZENA DAN TURUNANNYA
5.0 / 5 (0 votes)