Trick for Balancing Redox Reactions in Acidic Medium
Summary
TLDRIn this instructional video, the presenter outlines the steps for balancing redox reactions in acidic media. Key steps include dividing the reaction into oxidation and reduction half-reactions, balancing atoms (excluding hydrogen and oxygen), and then balancing oxygen with water and hydrogen with H⁺ ions. The video further explains how to equalize the number of electrons in each half-reaction before combining them into a balanced overall equation. Through a detailed example involving MnO₄⁻ and Fe²⁺, viewers learn a systematic approach to mastering redox reaction balancing, enhancing their understanding of this essential chemical concept.
Takeaways
- 😀 Understand the importance of dividing the redox reaction into two half-reactions based on oxidation and reduction.
- 🔍 Identify the oxidation and reduction processes by determining the changes in oxidation states of elements involved.
- ⚖️ Balance the number of atoms in the half-reactions, excluding hydrogen and oxygen, to ensure they match.
- 💧 For every oxygen atom in the reaction, add a water molecule to the opposite side to balance oxygen.
- 🧪 Add H⁺ ions to the opposite side for every excess hydrogen atom present in the reaction.
- 🔗 Ensure that the number of electrons lost in oxidation equals the number gained in reduction before combining half-reactions.
- ✖️ Use coefficients to multiply half-reactions as necessary to balance the number of electrons.
- 📊 Combine the balanced half-reactions to formulate the overall balanced redox reaction.
- 📝 An example involving MnO4⁻ and Fe²⁺ demonstrates the balancing process in acidic conditions.
- 👍 Encourage viewers to like, share, and subscribe for more educational content on balancing redox reactions.
Q & A
What is the first step in balancing a redox reaction?
-The first step is to divide the reaction into two half-reactions based on oxidation and reduction.
How do you identify which half-reaction undergoes oxidation?
-You identify the oxidation half-reaction by looking for the species that increases its oxidation state, indicating a loss of electrons.
What should be balanced after identifying the half-reactions?
-You should balance the number of atoms in each half-reaction, except for hydrogen and oxygen.
How is oxygen balanced in acidic media?
-In acidic media, each oxygen atom is balanced by adding one water molecule to the opposite side of the half-reaction.
What is the role of H⁺ ions in balancing hydrogen?
-For every excess hydrogen atom, H⁺ ions are added to the opposite side of the reaction to balance the hydrogen atoms.
Why is it important to balance the number of electrons in the half-reactions?
-Balancing the number of electrons ensures that the charge is equal on both sides of the overall reaction, allowing for accurate stoichiometry.
What do you do if the number of electrons in the half-reactions is not equal?
-If the number of electrons is not equal, you multiply the half-reactions by suitable coefficients to equalize the electrons before combining them.
Can you provide an example of a redox reaction involving manganese and iron?
-Yes, an example is the reduction of MnO₄⁻ to Mn²⁺ and the oxidation of Fe²⁺ to Fe³⁺.
What is the final balanced equation in the example given?
-The final balanced equation is MnO₄⁻ + 5 Fe²⁺ + 8 H⁺ → Mn²⁺ + 5 Fe³⁺ + 4 H₂O.
What should a student do if they find this balancing method helpful?
-Students are encouraged to like the video, share it, and subscribe to the channel for more educational content.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen

PENYETARAAN REAKSI REDOKS REAKSI REDOKS CARA BILANGAN OKSIDASI

Penyetaraan Reaksi Redoks Metode Perubahan Bilangan Oksidasi | Kimia Kelas 12
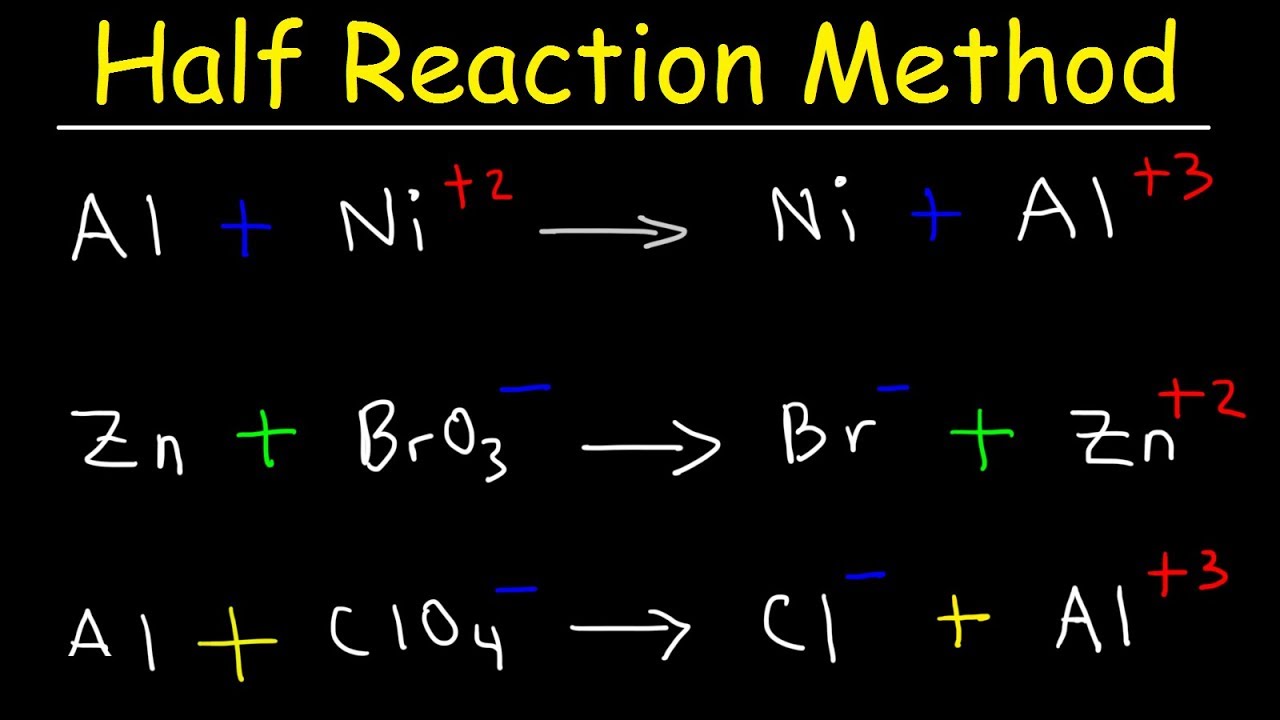
Half Reaction Method, Balancing Redox Reactions In Basic & Acidic Solution, Chemistry

Penyetaraan Reaksi Redoks Metode Bilangan Oksidasi | Kimia SMA | Tetty Afianti

Persamaan reaksi redoks

19.2 Balancing Redox Equations
5.0 / 5 (0 votes)
