ESTADOS DE OXIDACIÓN. REGLAS PARA ASIGNAR NUMEROS O ESTADOS DE OXIDACION
Summary
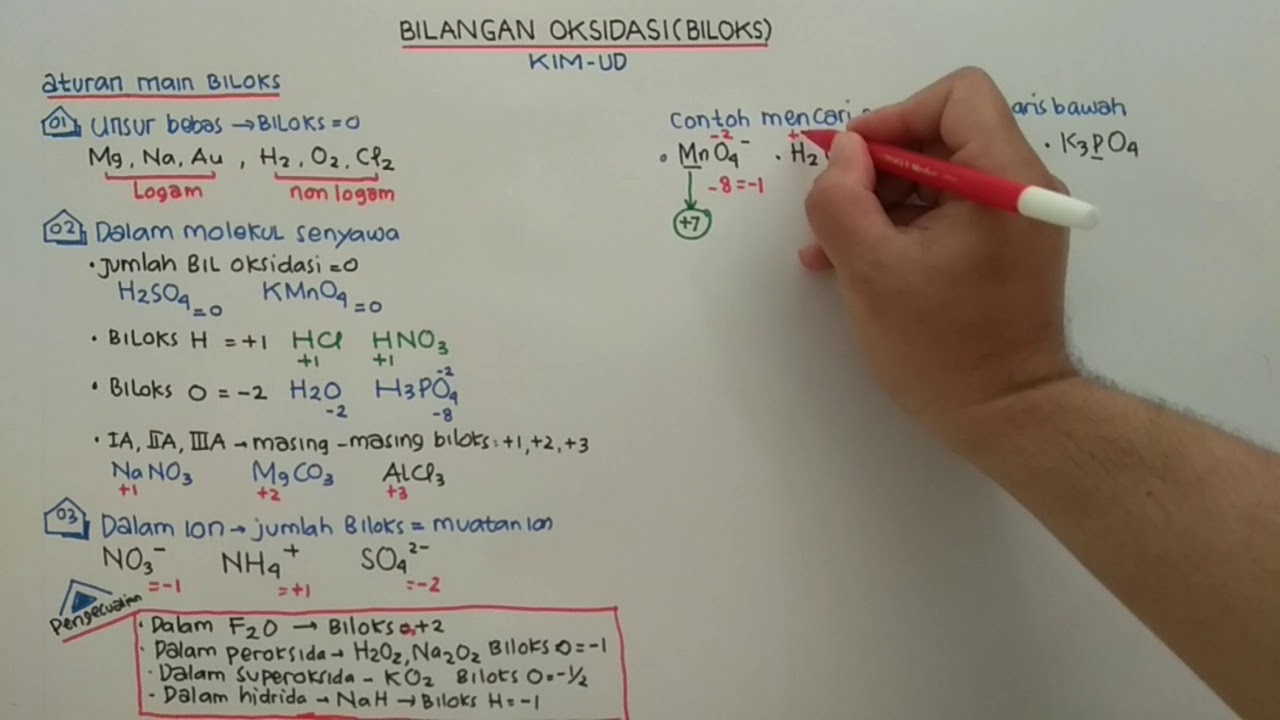
TLDRThe video introduces an easy-to-understand approach to learning oxidation states in chemistry. It begins by explaining the concept of oxidation numbers, which are crucial for naming inorganic compounds. The video covers five essential rules for assigning oxidation numbers, including how elements in a monoatomic state have an oxidation number of zero, and how hydrogen and oxygen generally have oxidation numbers of +1 and -2, respectively. The rules are applied to various examples, emphasizing their importance in understanding compound neutrality. The lesson is designed to be simple and useful for upcoming nomenclature topics.
Takeaways
- 😀 Oxidation states are essential for understanding the naming of inorganic compounds.
- ⚖️ Oxidation states are always integers, typically ranging from 1 to 7, and can be positive or negative.
- 🧠 Rule 1: The oxidation state of a monoatomic element is always zero when it is uncombined.
- 🔋 Rule 2: For group 1, 2, and 3 elements, the oxidation states are +1, +2, and +3 respectively when they are bonded.
- 🔑 Hydrogen generally has an oxidation state of +1, but it is -1 when combined with a metal (hydrides).
- 🌬️ Oxygen typically has an oxidation state of -2, except when combined with fluorine (+2) or in peroxides (-1).
- ⚙️ Rule 5: In compounds where the above rules don't apply, the oxidation states are assigned based on neutrality—positive and negative charges should cancel out to zero.
- 💡 To determine an unknown oxidation state, balance the known oxidation states of other elements in the compound.
- 🔍 Example: In NaCl, sodium (Na) has an oxidation state of +1, and chlorine (Cl) has -1, balancing each other out.
- 🧪 Correct application of oxidation states is essential for compound neutrality, with positive and negative charges always balancing.
Q & A
What are oxidation states?
-Oxidation states represent the net charge an atom would have if electrons were completely transferred to the more electronegative atom in a bond. This concept helps in understanding how compounds are named and their chemical behavior.
What are the typical oxidation states for elements in the periodic table?
-Elements in groups 1, 2, and 3 typically have oxidation states of +1, +2, and +3, respectively. For example, sodium has +1, calcium has +2, and aluminum has +3.
What is the oxidation state of an element in its monatomic form?
-The oxidation state of an element in its monatomic form (e.g., sodium or oxygen molecules) is always zero.
How do you assign oxidation states to hydrogen?
-Hydrogen usually has an oxidation state of +1 when bonded with nonmetals. However, when bonded with metals, as in hydrides, it has an oxidation state of -1.
What are the rules for assigning oxidation states to oxygen?
-Oxygen typically has an oxidation state of -2, except when bonded to fluorine (where it is +2) or in peroxides (where it is -1).
What is the sum of oxidation states in a neutral molecule?
-In a neutral molecule, the sum of the oxidation states of all atoms must equal zero to ensure that the molecule is electrically neutral.
How do you calculate the oxidation state of chlorine in NaCl?
-In NaCl, sodium has an oxidation state of +1, so chlorine must have an oxidation state of -1 to balance the charges and maintain a neutral molecule.
Why is it important to balance oxidation states in molecules?
-Balancing oxidation states ensures that the total charges of a molecule or compound are neutral, following the rule that compounds must have no overall charge unless they are ions.
How does oxidation state assignment help with chemical nomenclature?
-Understanding oxidation states is crucial for naming inorganic compounds correctly, as it indicates the charge of each element and helps identify the compound's structure.
What is a key takeaway for mastering oxidation states?
-A solid grasp of the rules for assigning oxidation states, especially for common elements like oxygen and hydrogen, is essential for understanding chemical reactions and naming compounds.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführen5.0 / 5 (0 votes)