Penentuan Bilangan Oksidasi: Materi Kimia Kelas X
Summary
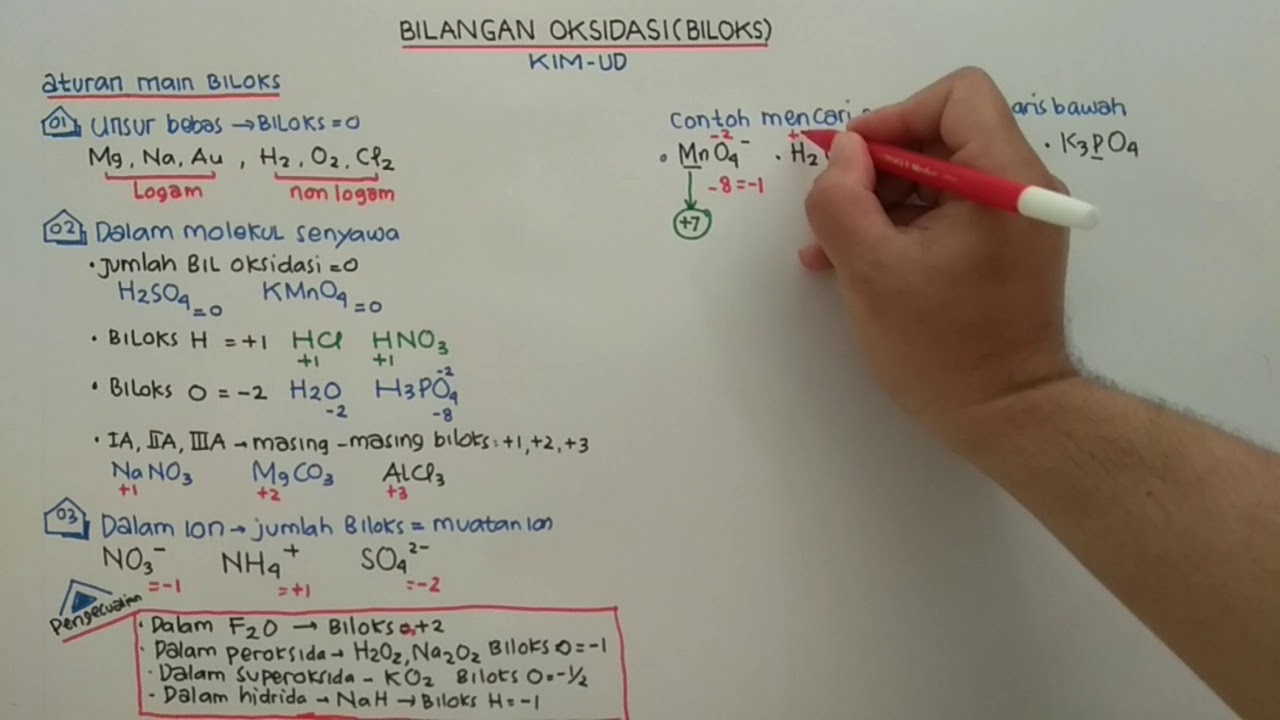
TLDRThis chemistry tutorial explains the concept of oxidation states (bilangan oksidasi), focusing on how to determine them for elements, ions, and compounds. The video covers key rules, such as oxidation states of metals in groups 1A, 2A, and 3A, ion charges, and specific rules for oxygen and hydrogen. It demonstrates how to apply these rules through examples like MnSO4 and MNO4-, helping students understand oxidation-reduction reactions. With clear explanations and step-by-step exercises, this video serves as a useful guide for mastering oxidation state calculations in chemistry.
Takeaways
- 😀 Oxidation numbers (bilangan oksidasi) are used to represent the ability of a substance to gain or lose electrons, with positive values indicating electron loss and negative values indicating electron gain.
- 😀 Redox reactions involve both reduction (decrease in oxidation number) and oxidation (increase in oxidation number).
- 😀 The oxidation number of alkali metals (Group 1A) is always +1, alkaline earth metals (Group 2A) is +2, and Group 3A metals like aluminum have an oxidation number of +3.
- 😀 For ions, the oxidation number is equal to the ion's charge. For example, Na+ has an oxidation number of +1, and Cl- has an oxidation number of -1.
- 😀 Free elements (such as H2, O2, N2) have an oxidation number of zero.
- 😀 In a compound or molecule, the sum of oxidation numbers of all atoms must equal zero (for neutral compounds) or the overall charge (for ions).
- 😀 Sulfur (S) in the sulfate ion (SO4^2-) has an oxidation number of +6, while oxygen (O) typically has an oxidation number of -2.
- 😀 Oxygen generally has an oxidation number of -2, except in peroxides (where it is -1) and in OF2 (where it is +2).
- 😀 Hydrogen usually has an oxidation number of +1, except when it is part of hydrides with metals where it is -1.
- 😀 Understanding oxidation numbers requires familiarity with a set of rules and the ability to apply them in specific contexts, like determining the oxidation state in compounds and ions.
Q & A
What is the definition of oxidation in the context of redox reactions?
-Oxidation is the reaction where the oxidation number of a substance increases, meaning it loses electrons.
What is the definition of reduction in redox reactions?
-Reduction is the reaction where the oxidation number of a substance decreases, meaning it gains electrons.
What is an oxidation number (bilangan oksidasi or biloks)?
-An oxidation number is a measure of a substance's ability to gain or lose electrons, and it is expressed as a number that can be positive or negative.
How do you determine the oxidation number of a Group 1A metal?
-The oxidation number of a Group 1A metal is equal to its group number. For example, sodium (Na) has an oxidation number of +1.
What is the oxidation number of oxygen in most compounds?
-In most compounds, the oxidation number of oxygen is -2, except in peroxides (where it is -1) and in OF₂ (where it is +2).
What rule applies to the oxidation number of an ion?
-The oxidation number of an ion is equal to its charge. For example, Na⁺ has an oxidation number of +1, and Cl⁻ has an oxidation number of -1.
What is meant by 'free elements' in the context of oxidation numbers?
-Free elements are atoms that are not bonded to other atoms, and their oxidation number is 0. Examples include O₂, N₂, and H₂.
How can you determine the oxidation number of sulfur in the ion SO₄²⁻?
-In the ion SO₄²⁻, the oxidation number of oxygen is -2. Since there are four oxygen atoms, the total oxidation number of oxygen is -8. To balance the charge of -2 for the ion, the oxidation number of sulfur (S) must be +6.
What is the oxidation number of hydrogen in most compounds?
-In most compounds, the oxidation number of hydrogen is +1, except when it is part of a metal hydride, where it is -1.
How would you determine the oxidation number of manganese (Mn) in MnSO₄?
-In MnSO₄, the oxidation number of sulfur (S) is +6, and the oxidation number of oxygen is -2. Since the compound is neutral, the oxidation number of Mn must be +2 to balance the charges.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)