Difference between Atoms and Ions (Explanation & Examples)
Summary
TLDRThis video explains the key differences between atoms and ions, focusing on the role of electrons. Atoms are neutral, having equal protons and electrons, while ions have an imbalance due to gaining or losing electrons. The video uses examples like chlorine and nitrogen to show how atoms become negatively or positively charged ions through chemical bonding, particularly in ionic bonds. It also touches on periodic table trends, cations (positive ions), anions (negative ions), and polyatomic ions, making complex concepts simpler with clear visual examples.
Takeaways
- 🔬 Atoms and ions differ in the number of electrons, with ions having a charge due to an imbalance between protons and electrons.
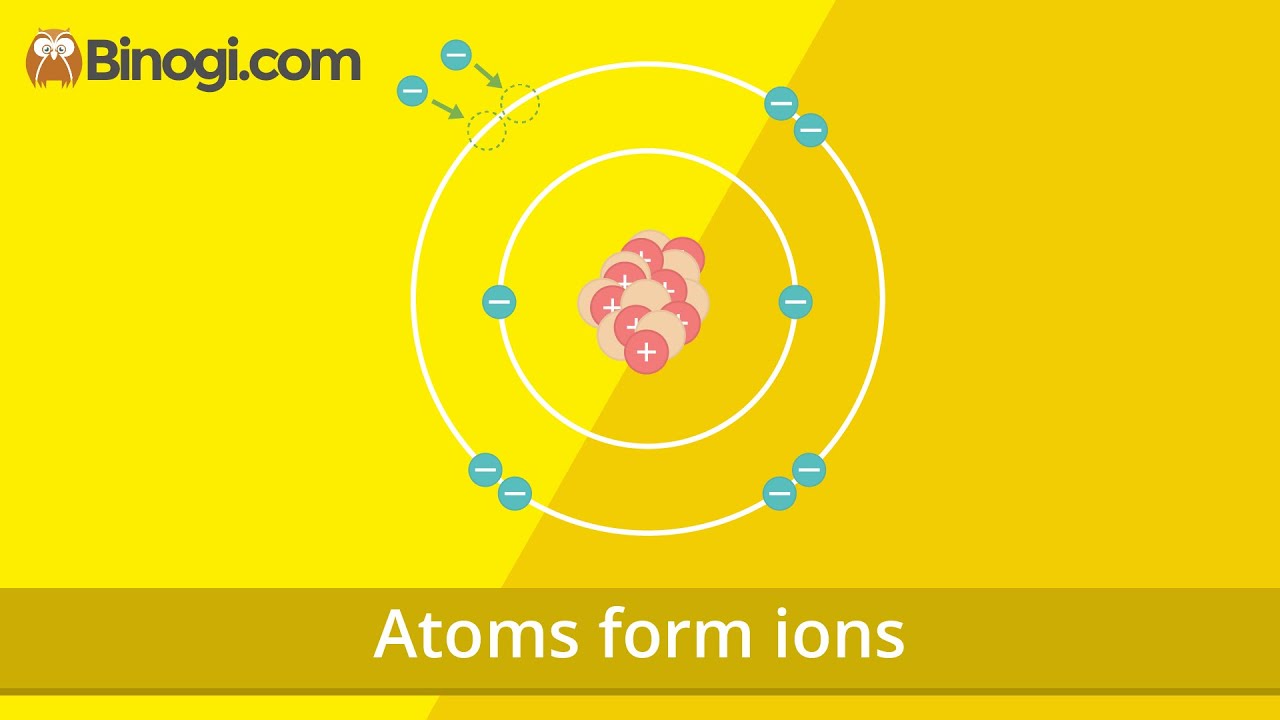
- 🧪 Chlorine (Cl) is neutral in its atomic form, with equal numbers of protons and electrons.
- ⚛️ When chlorine gains an electron during chemical bonding, it becomes a negatively charged ion (Cl⁻).
- 🔋 Nitrogen, with an atomic number of 7, has 7 protons and 7 electrons in its neutral state.
- 💡 Ions are charged particles because the number of electrons does not equal the number of protons.
- ⚠️ Adding neutrons to the nucleus changes the atomic mass but not the charge of the atom or ion.
- ⚖️ Neutral atoms have equal numbers of protons and electrons, while ions do not.
- 📉 Nitrogen typically forms a 3⁻ ion because it gains 3 electrons to fill its outer shell.
- 🧲 Atoms that lose or gain electrons become ions and can form ionic bonds due to opposite charges (e.g., Na⁺ and Cl⁻).
- 📛 Cations are positively charged ions (e.g., Na⁺), while anions are negatively charged ions (e.g., Cl⁻).
Q & A
What is the main difference between an atom and an ion?
-The main difference between an atom and an ion is the number of electrons. An atom is neutral, with an equal number of protons and electrons, while an ion has either gained or lost electrons, resulting in a charge.
Why is chlorine (Cl) considered a neutral atom?
-Chlorine (Cl) is considered a neutral atom because the number of protons equals the number of electrons, which balances the positive and negative charges.
What happens when chlorine forms an ionic bond?
-When chlorine forms an ionic bond, it gains an extra electron, resulting in a negative charge, making it a negatively charged ion (Cl⁻).
How do protons and electrons affect the charge of an atom or ion?
-Protons are positively charged, and electrons are negatively charged. If an atom has an equal number of protons and electrons, it is neutral. If an atom has more electrons than protons, it becomes a negatively charged ion, and if it has fewer electrons, it becomes a positively charged ion.
What is the charge of a nitrogen ion, and why?
-A nitrogen ion typically has a charge of 3⁻ because it gains three extra electrons to fill its outer shell, resulting in three more negative charges than positive protons.
How does the addition of neutrons affect the charge of an atom?
-The addition of neutrons does not affect the charge of an atom. Neutrons are neutral particles, so they only change the atomic mass, not the charge.
What is the general trend for ions in Group 1 and Group 2 elements of the periodic table?
-Group 1 elements, like sodium (Na), tend to lose one electron to form 1⁺ ions. Group 2 elements lose two electrons to form 2⁺ ions.
How are positive and negative ions (cations and anions) formed?
-Positive ions (cations) are formed when an atom loses electrons, making it positively charged. Negative ions (anions) form when an atom gains electrons, giving it a negative charge.
What is an ionic bond, and how does it form between sodium and chlorine?
-An ionic bond is the attraction between oppositely charged ions. It forms when sodium (Na) loses an electron to become Na⁺, and chlorine (Cl) gains that electron to become Cl⁻, resulting in the attraction between Na⁺ and Cl⁻ to form sodium chloride (NaCl).
What are polyatomic ions, and how do they get their charge?
-Polyatomic ions are groups of atoms bonded together that have an overall positive or negative charge. They gain or lose electrons from other atoms, resulting in their charge.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen
5.0 / 5 (0 votes)