Physical Science made Easy by SAM Animation
Summary
TLDRThis lesson from Crosby’s class dives into the structure of atoms, explaining how everything in the world is made up of them. The focus is on electrons and their organization into sublevels (s, p, d, f), the quantum model of the atom, and electron configuration using the diagonal rule. Through examples like magnesium and chlorine, the lesson shows how atoms bond through ionic bonding. Magnesium donates electrons to chlorine, stabilizing both elements, forming magnesium chloride. The script also touches on practical applications of magnesium chloride, including its use in tofu production and as an anti-ice agent.
Takeaways
- 🔬 Atoms make up everything in the world, including everyday objects like cell phones.
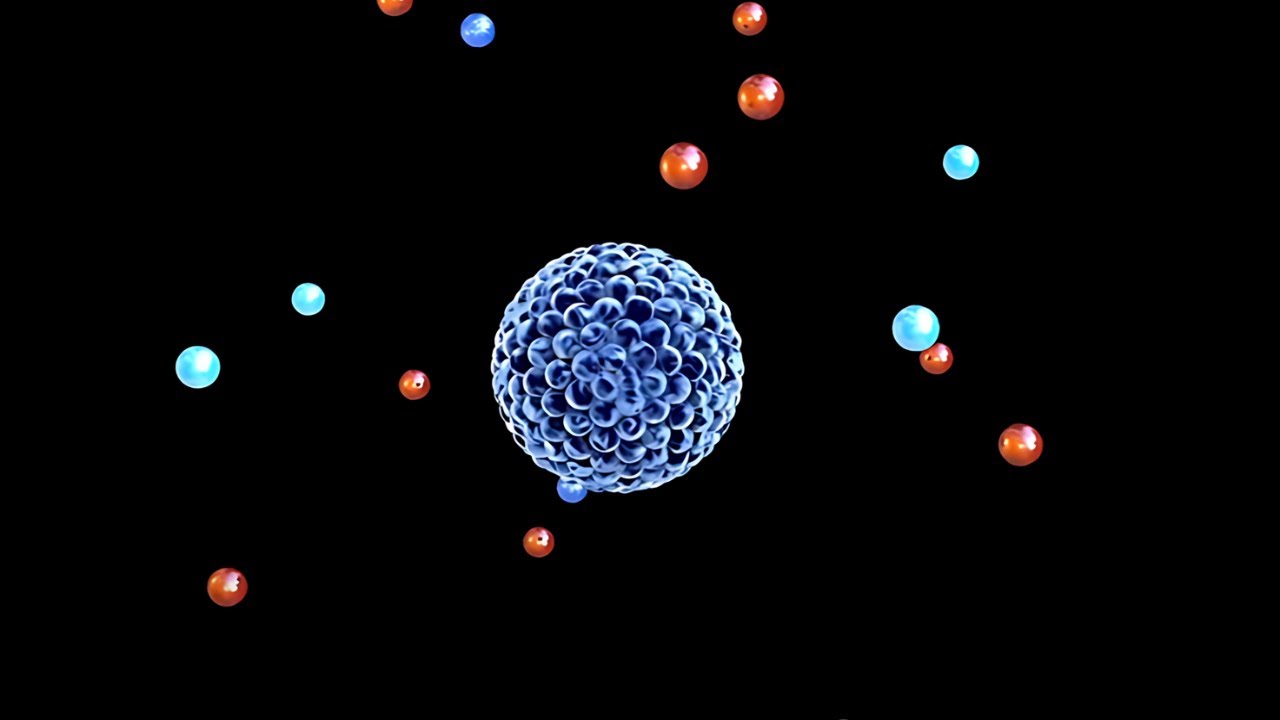
- 🧪 Atoms consist of a nucleus with protons (positive charge), neutrons (no charge), and electrons (negative charge) that orbit the nucleus.
- 📊 Electrons occupy specific sublevels called s, p, d, and f, with each sublevel holding a different number of electrons: s holds 2, p holds 6, d holds 10, and f holds 14.
- 🔭 The quantum model of the atom is based on the x, y, and z axes to represent three dimensions.
- ⚛️ Energy levels are filled according to the diagonal rule, where electrons fill the lowest available energy levels first.
- 🔋 Magnesium, with 12 electrons, follows the diagonal rule for electron configuration, filling sublevels in the order 1s, 2s, 2p, and 3s.
- 🧩 Chlorine has 17 electrons, and its outermost sublevel (3p) is not fully filled, making it less stable and reactive.
- 💡 Valence electrons are the outermost electrons, which play a key role in chemical bonding.
- ⚙️ Magnesium and chlorine form an ionic bond where magnesium donates its valence electrons to chlorine, resulting in stable ions.
- 🧂 The ionic compound formed between magnesium and chlorine is called magnesium chloride (MgCl2), and it is used in processes like making tofu and as an anti-ice agent.
Q & A
What are atoms made of?
-Atoms are made of a nucleus containing positively charged protons and neutrons with no charge. Electrons, which have a negative charge, orbit the nucleus.
What are the four sublevels in which electrons are arranged?
-The four sublevels where electrons are arranged are s, p, d, and f.
How many electrons can each sublevel hold?
-The s sublevel can hold 2 electrons, the p sublevel can hold 6, the d sublevel can hold 10, and the f sublevel can hold 14 electrons.
What is the diagonal rule in electron configuration?
-The diagonal rule shows how electrons fill energy levels from the lowest to the highest, with each higher level having one more sublevel than the previous. Electrons fill these levels in a specific order as indicated by the red diagonal arrows.
How is the electron configuration of magnesium written?
-Magnesium has 12 electrons, so following the diagonal rule, its electron configuration is 1s² 2s² 2p⁶ 3s².
What are valence electrons?
-Valence electrons are the electrons in the outermost energy level of an atom. They are responsible for chemical bonding.
How does the electron configuration of chlorine differ from magnesium?
-Chlorine has 17 electrons, and while the first two energy levels are similar to magnesium, chlorine has five additional electrons in its 3p orbital. This makes chlorine less stable as its outer level is not fully filled.
What is ionic bonding, and how does it work between magnesium and chlorine?
-Ionic bonding occurs when magnesium, which has two valence electrons it wants to give away, transfers those electrons to two chlorine atoms, which need one electron each to be stable. Magnesium becomes a positive ion, and chlorine becomes a negative ion, forming an ionic bond.
What is magnesium chloride's empirical formula, and what does it represent?
-Magnesium chloride's empirical formula is MgCl₂, representing the ratio of atoms in the compound, with one magnesium atom bonding with two chlorine atoms.
What are some uses of magnesium chloride?
-Magnesium chloride is used in making tofu, soy milk, and as an anti-ice agent. Although not for consumption, it is non-toxic and non-flammable.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenWeitere ähnliche Videos ansehen

The Crazy Mass-Giving Mechanism of the Higgs Field Simplified

What is the universe made of? - Dennis Wildfogel

Proteínas e Aminoácidos - Aula 07 - Módulo I: Biologia Celular | Prof. Guilherme

How Small Is An Atom? Spoiler: Very Small.

Bill Nye The Science Guy Atoms & Molecules
INILAH PARTIKEL DASAR PENYUSUN ATOM
5.0 / 5 (0 votes)
