VIDEO PEMBELAJARAN - FISIKA - HUKUM I TERMODINAMIKA
Summary
TLDRIn this educational video, the host, Kak Irma Lismayani, introduces the topic of thermodynamics, focusing on its first law. The law is explained through simple examples, such as boiling water, to demonstrate how energy transfer as heat or work affects a system. Key concepts like system, surroundings, and internal energy changes are explored. The video emphasizes that thermodynamics plays a role in everyday life, from cooking to human metabolism. The host encourages students to grasp the fundamental concepts of energy conservation, providing both theoretical and practical insights for understanding thermodynamics.
Takeaways
- 😀 Termodynamics is a branch of physics that studies the basic laws of energy transfer in the form of heat and work.
- 😀 The difference between heat and work: Heat transfers energy due to temperature differences, while work does not depend on temperature differences.
- 😀 A 'system' refers to the object or subject of observation, and the 'environment' refers to everything outside the system that can affect it.
- 😀 The first law of thermodynamics explains that heat added to a system can be used to do work or increase internal energy.
- 😀 The equation for the first law of thermodynamics is Q = ΔU + W, where Q is heat, ΔU is the change in internal energy, and W is work.
- 😀 Positive Q means heat is added to the system, while negative Q means the system releases heat.
- 😀 Work (W) is positive when the system does work, and negative when work is done on the system.
- 😀 The change in internal energy (ΔU) is positive if the system gains energy and negative if it loses energy.
- 😀 An example from everyday life: when boiling water, heat is added to the system (water), causing a change in internal energy and work in the form of vaporizing water.
- 😀 In real life, thermodynamics applies to various processes such as human metabolism, where the body converts energy to perform work and maintain its functions.
Q & A
What is thermodynamics, and what does it study?
-Thermodynamics is a branch of physics that studies the basic laws of energy transfer in the form of heat and work. It focuses on how energy moves within systems and the changes that occur as a result of these energy transfers.
What are the two key concepts in thermodynamics?
-The two key concepts in thermodynamics are 'system' and 'environment'. A system refers to the object or state being observed, while the environment encompasses everything outside the system that can influence its state.
Can you give an example of a system and its environment?
-An example of a system is the air inside a room, while the environment would be the air outside the room or sunlight coming from outside. The environment can affect the system's state, like temperature or pressure.
What does the First Law of Thermodynamics state?
-The First Law of Thermodynamics states that when heat is added to a system, it can either be used to do work or result in a change in the internal energy of the system. Mathematically, it's expressed as Q = ΔU + W, where Q is heat, ΔU is the change in internal energy, and W is the work done.
What happens when you heat water in a pot according to thermodynamics?
-When you heat water in a pot, you add heat (energy) to the water, causing its internal energy to increase. As the water absorbs heat, it can do work, such as causing the lid to move or steam to escape.
What do the symbols Q, ΔU, and W represent in the First Law of Thermodynamics?
-In the First Law of Thermodynamics, Q represents heat (calories or joules), ΔU represents the change in internal energy of the system, and W represents the work done by the system.
How do you calculate the change in internal energy of a system?
-To calculate the change in internal energy (ΔU), you use the equation ΔU = Q - W, where Q is the heat added to the system, and W is the work done by the system.
In what scenario would the change in internal energy be negative?
-The change in internal energy would be negative if the system loses energy, such as when heat is released or when the system does work on the surroundings.
How do positive and negative signs affect the interpretation of the First Law of Thermodynamics?
-A positive Q means heat is added to the system, a negative Q means heat is released by the system. A positive W means the system does work, and a negative W means the system receives work. Similarly, a positive ΔU means the system gains energy, while a negative ΔU means the system loses energy.
Can the First Law of Thermodynamics be applied to the human body?
-Yes, the First Law of Thermodynamics can be applied to the human body. When humans perform physical activities, they use energy (work), and their internal energy decreases. To maintain body temperature, heat is transferred from the body to the environment, illustrating the concepts of work and heat transfer in thermodynamics.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة

Pendahuluan - Perpindahan Kalor dan Massa
12th Physics | Chapter 4 | Thermodynamics | Lecture 2 | Maharashtra Board |

1ª LEI DA TERMODINÂMICA | Resumo de Física para o Enem
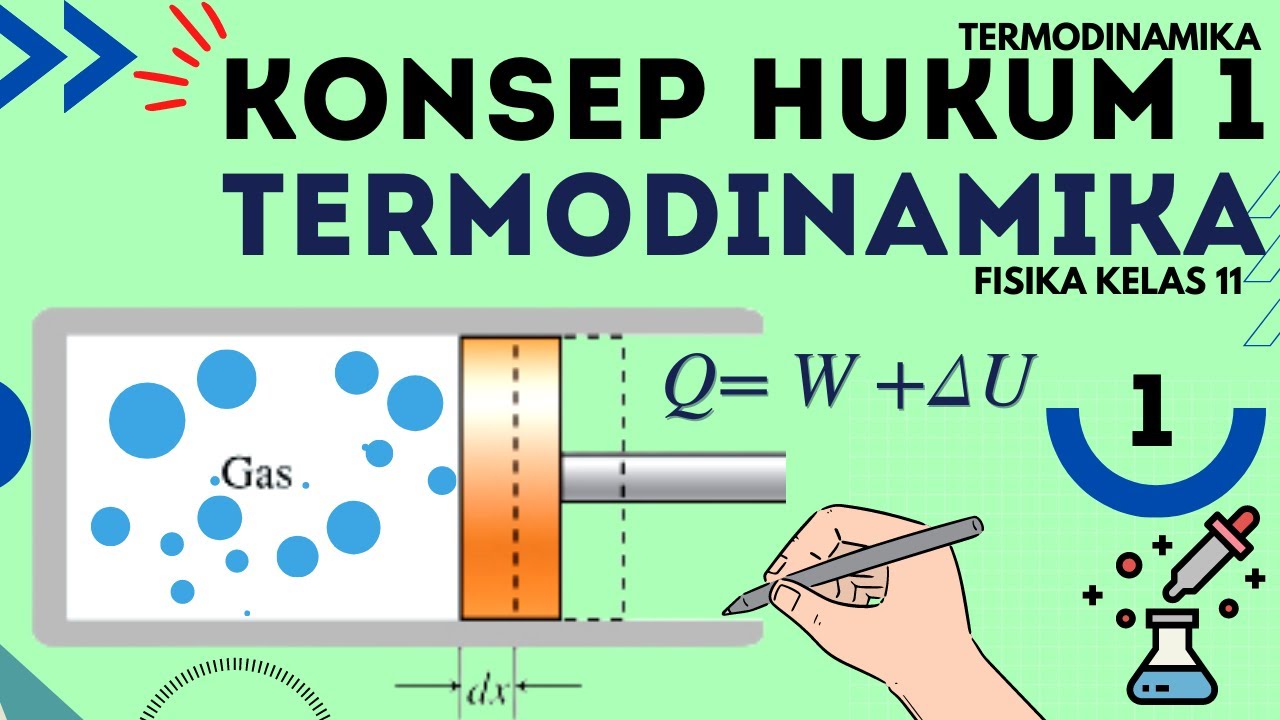
Hukum 1 Termodinamika : Termodinamika Fisika Kelas 11 - Part 1

Ciri-Ciri Virus dan Bentuk Virus Kelas 10 | Materi Biologi UTBK

Kelas 3 Tematik : Tema 7 Subtema 1 Pembelajaran 1 (PERKEMBANGAN TEKNOLOGI)
5.0 / 5 (0 votes)
