I never understood why too many neutrons cause instability - until now!
Summary
TLDRThis video explores the intriguing topic of nuclear stability, focusing on why elements like Iron-56 are the most stable and how this affects our existence. It delves into the interplay of the strong nuclear force and electrostatic repulsion, explaining how the addition of neutrons and protons influences stability. Key concepts such as beta and alpha decay are highlighted, showing how nature manages unstable nuclei. The discussion also touches on how stellar fusion creates heavier elements, ultimately providing a foundation for life. The integration of Squarespace is used as a metaphor for building stability, both in websites and atomic nuclei.
Takeaways
- 😀 The most stable elements in the universe, like Iron-56, have high binding energy per nucleon, but this alone doesn't explain why nuclei are stable or unstable.
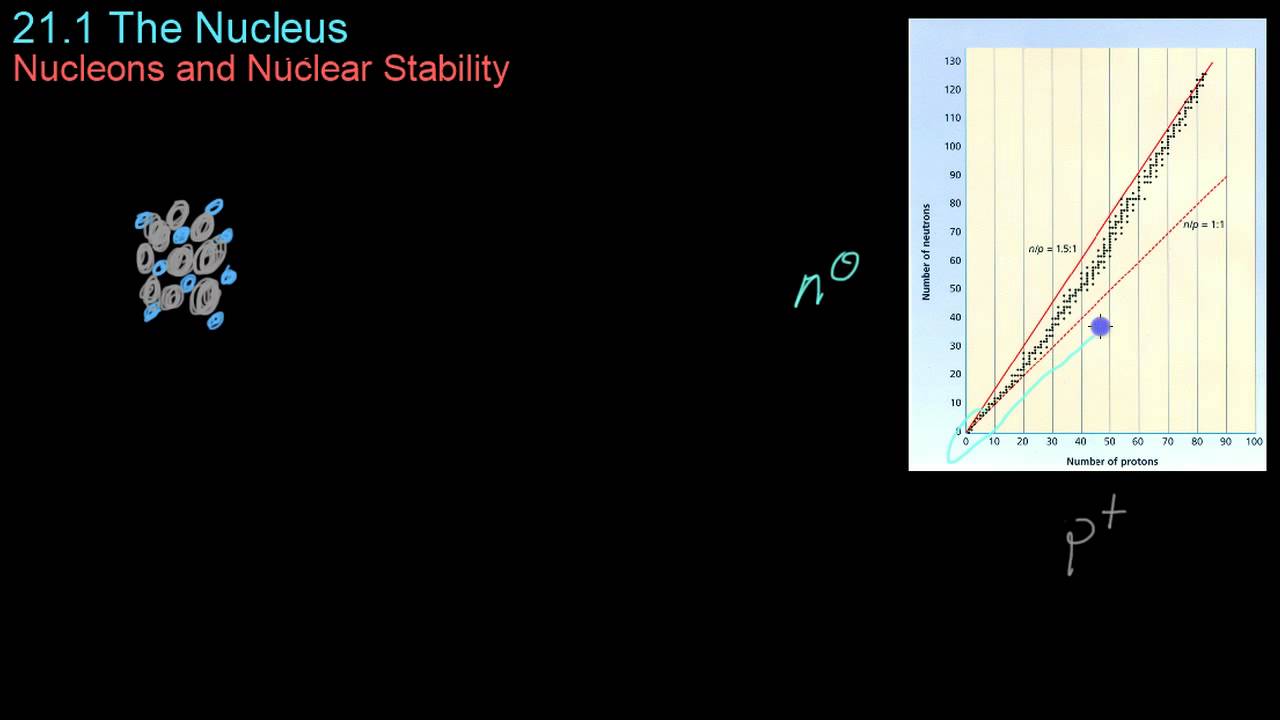
- 😀 Initially, heavier nuclei are more stable due to the increased strong nuclear force between particles, but beyond a certain size, the stability decreases due to overwhelming Coulomb repulsion.
- 😀 The balance between protons' mutual repulsion and neutrons' stabilizing role is crucial in determining nuclear stability.
- 😀 Nature uses beta decay (β- and β+ decay) to convert neutrons to protons and vice versa, helping stabilize unstable nuclei.
- 😀 Heavier nuclei require more neutrons than protons for stability, as neutrons help counteract the increasing Coulomb repulsion between protons in large nuclei.
- 😀 Nuclei like helium-16 are unstable because neutrons occupy higher energy levels, and nature uses beta decay to convert them into protons for stability.
- 😀 Stability in atomic nuclei is not just about the number of protons and neutrons, but how their energy levels and quantum states interact.
- 😀 Po’s Exclusion Principle explains why electrons and nucleons (protons and neutrons) cannot occupy the same energy state, influencing the structure of stable nuclei.
- 😀 As protons and neutrons increase in number, the strong nuclear force increases the stability of the nucleus up to a point, after which the nuclear force becomes saturated and Coulomb repulsion dominates.
- 😀 In large nuclei, the need for more neutrons increases, but nature doesn’t convert high-energy neutrons into protons, which leads to instability as the system becomes less stable.
- 😀 Alpha decay (emission of helium nuclei) is a process through which large unstable nuclei release excess energy and reduce proton repulsion, stabilizing the system.
Q & A
Why is Iron-56 considered one of the most stable elements in the universe?
-Iron-56 is considered highly stable because it has a very high binding energy per nucleon. This means it is tightly bound and difficult to break apart, contributing to its stability.
What happens to the stability of nuclei as they become heavier?
-Initially, as nuclei become heavier, their stability increases. However, after a certain point, the stability decreases as the nucleus becomes too large for the strong nuclear force to counterbalance the electrostatic repulsion between protons.
Why does the graph of nuclear stability first increase and then decrease as the number of protons and neutrons increases?
-The graph of nuclear stability increases because the strong nuclear force becomes more effective at binding protons and neutrons as the number of particles increases. However, beyond a certain size, the electrostatic repulsion between protons outweighs the strong nuclear force, leading to instability.
How does nuclear fusion contribute to the creation of elements, and why does it stop at Iron-56?
-Nuclear fusion powers stars by fusing lighter elements into heavier ones. This process continues until Iron-56 is formed because fusing iron further would require energy, rather than releasing it, making fusion beyond iron unsustainable.
How do neutrons contribute to the stability of atomic nuclei?
-Neutrons help stabilize atomic nuclei by counteracting the electrostatic repulsion between protons. Their presence helps to balance the forces within the nucleus, ensuring that the nucleus remains stable.
Why does a nucleus with only protons and no neutrons (e.g., Helium-16) tend to be unstable?
-Helium-16 would be unstable because, without neutrons, the protons would experience excessive electrostatic repulsion from each other. Neutrons help mitigate this repulsion by adding strong nuclear force without contributing to the repulsion.
What is Po's Exclusion Principle, and how does it apply to protons and neutrons?
-Po's Exclusion Principle states that no two particles can occupy the same quantum state. For protons and neutrons, this means they must occupy discrete energy levels, with neutrons occupying higher levels than protons in a stable nucleus.
What is beta decay, and how does it relate to nuclear stability?
-Beta decay is a process where neutrons or protons are converted into each other, releasing either an electron (beta minus decay) or a positron (beta plus decay). This process allows nuclei to adjust the ratio of protons to neutrons, helping them reach a more stable configuration.
Why do heavier elements require more neutrons than lighter elements to remain stable?
-Heavier elements require more neutrons because the increasing number of protons causes stronger electrostatic repulsion, which must be balanced by additional neutrons. These neutrons help bind the nucleus together and prevent it from becoming unstable.
How does the strong nuclear force compare to the electrostatic force in large nuclei?
-The strong nuclear force is much stronger than the electrostatic force at short distances, binding protons and neutrons together. However, because the strong force has a limited range, in large nuclei, the electrostatic repulsion between protons eventually overwhelms the nuclear attraction, leading to instability.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
5.0 / 5 (0 votes)