Termodinamika: Cabang Fisika yang Luar Biasa
Summary
TLDRThis video explores the fascinating world of thermodynamics, a field of science that studies the relationship between energy, heat, and work. It explains fundamental concepts like temperature, the Zeroth Law of Thermodynamics, and the First and Second Laws, which govern energy conservation and entropy. Through relatable examples such as how refrigerators, car engines, and household appliances work, the video highlights the importance of thermodynamics in both daily life and technological advancements. The discussion emphasizes the role of thermodynamics in improving energy efficiency and fostering innovation in various industries, making it an essential part of modern technology and science.
Takeaways
- 😀 Thermodynamics explains how heat, energy, and work are interrelated and affects everything from household appliances to car engines.
- 😀 Temperature is a measure of the average kinetic energy of particles in a substance, affecting how hot or cold something feels.
- 😀 The Zeroth Law of Thermodynamics establishes the concept of thermal equilibrium: if two systems are in equilibrium with a third, they are in equilibrium with each other.
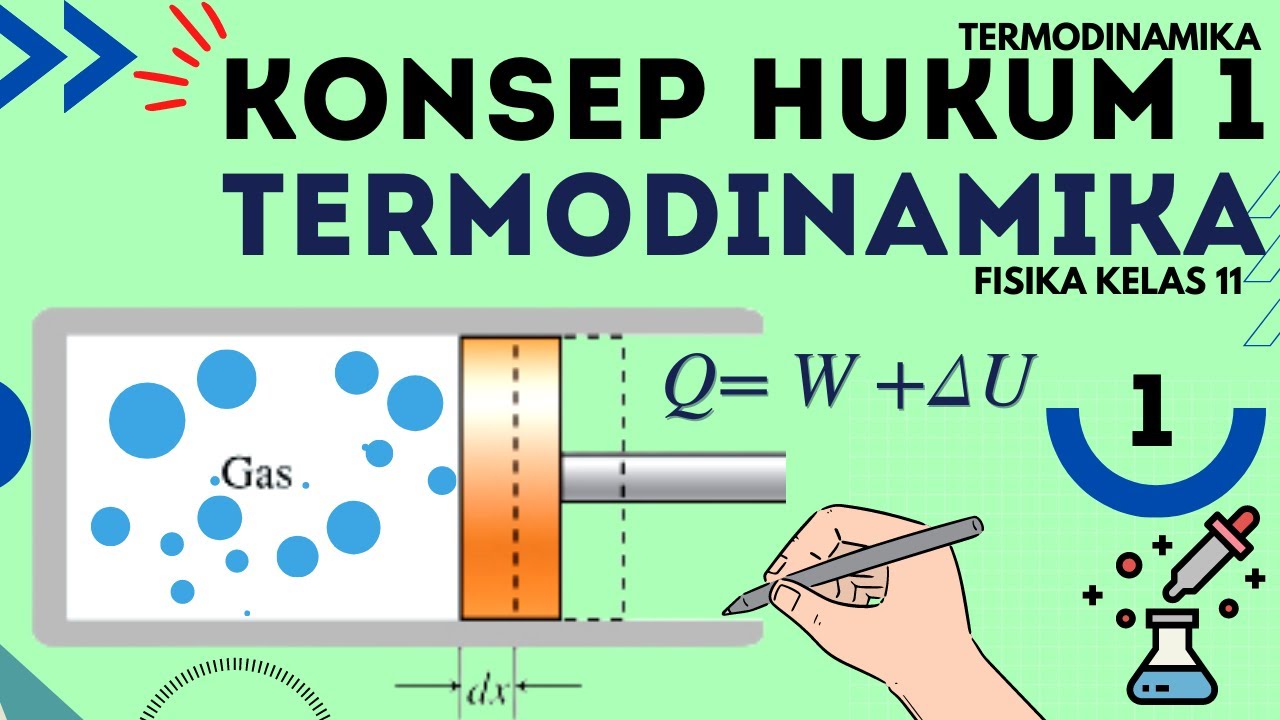
- 😀 The First Law of Thermodynamics, also known as the Law of Energy Conservation, states that energy cannot be created or destroyed, only converted from one form to another.
- 😀 An example of the First Law: in a bike pump, muscle energy is transferred to air, increasing its internal energy and causing temperature and pressure to rise.
- 😀 The Second Law of Thermodynamics introduces the concept of entropy, which always increases in isolated systems over time, signifying that energy tends to spread out.
- 😀 The Second Law also highlights the irreversibility of certain processes, such as heat flowing from hot to cold.
- 😀 Thermodynamics plays a crucial role in everyday technology, such as car engines, refrigerators, air conditioners, and water heaters, by managing heat transfer effectively.
- 😀 Sustainable energy development is essential because, according to the First and Second Laws, energy cannot be created from nothing and will always spread out, making efficiency key.
- 😀 The laws of thermodynamics have far-reaching implications for technology and innovation, influencing industries like energy production, transportation, and material science.
Q & A
What is thermodynamics?
-Thermodynamics is the scientific study of energy and its transformations, focusing on the relationship between heat, work, temperature, and energy. It explains how energy changes from one form to another, from atomic scales to the scale of the universe.
How does thermodynamics apply to everyday technologies?
-Thermodynamics is essential in understanding how machines and household appliances function. For example, in engines, thermodynamics explains how chemical energy from fuel is converted into heat and mechanical work. Similarly, refrigerators, air conditioners, and water heaters rely on thermodynamic principles to transfer heat efficiently.
What does the Zeroth Law of Thermodynamics state?
-The Zeroth Law of Thermodynamics states that if two systems are each in thermal equilibrium with a third system, they are also in thermal equilibrium with each other. This principle establishes a foundation for all other thermodynamic laws.
What is the First Law of Thermodynamics?
-The First Law of Thermodynamics, or the law of conservation of energy, states that energy cannot be created or destroyed, only transformed from one form to another. For example, in a bicycle pump, muscle energy is transferred to the air inside the pump, raising its internal energy and temperature.
What does the Second Law of Thermodynamics explain?
-The Second Law of Thermodynamics states that the total entropy of an isolated system can never decrease over time. It describes how energy tends to spread out and become more evenly distributed, making certain processes irreversible, like heat flowing from a hot object to a colder one.
How does thermodynamics relate to engine efficiency?
-Thermodynamics is key to understanding engine performance. For example, in an internal combustion engine, chemical energy from fuel is converted to heat, which is then transformed into mechanical work that drives the car. Optimizing this conversion process is crucial for improving fuel efficiency.
Why is understanding thermodynamics important for renewable energy technologies?
-Understanding thermodynamics helps in designing efficient energy systems. The laws of thermodynamics emphasize the importance of sustainable energy solutions, as energy conversion and transformation need to be carefully managed to reduce waste and maximize the use of renewable resources like solar, wind, and hydro power.
What role does entropy play in thermodynamics?
-Entropy is a measure of disorder or randomness in a system. The Second Law of Thermodynamics asserts that the entropy of an isolated system always increases over time, meaning energy tends to disperse and become less concentrated, which is why certain processes are irreversible.
What practical examples of thermodynamics can we find in daily life?
-Practical examples of thermodynamics in daily life include household appliances like refrigerators, air conditioners, and water heaters. These devices use thermodynamic cycles to move heat from one place to another, making our homes more comfortable.
How does thermodynamics influence technological innovation?
-Thermodynamics plays a crucial role in driving technological innovations. By understanding energy transformations, scientists and engineers can design more efficient machines, develop better materials, and create energy systems that minimize waste and improve performance, thereby advancing technology in fields like electricity generation and materials science.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
5.0 / 5 (0 votes)