Gibbs Free Energy
Summary
TLDRThis video explains the key concepts of entropy, enthalpy, and Gibbs Free Energy in relation to spontaneous processes. It highlights how entropy (ΔS) measures disorder, and how enthalpy (ΔH) and temperature (T) affect the spontaneity of reactions. The video introduces Gibbs Free Energy (ΔG) as the definitive indicator of spontaneity, explaining that if ΔG is negative, the process is spontaneous. Various scenarios are discussed, including the effects of positive and negative ΔH and ΔS on spontaneity. The video concludes with a numerical example, illustrating the calculation of the temperature required for a reaction to become spontaneous.
Takeaways
- 😀 Entropy (ΔS) measures the degree of disorder in a system. A spontaneous process increases the disorder (entropy).
- 😀 Entropy is positive when the disorder in a system increases. A gas particle spreading out in a container is an example of this.
- 😀 Enthalpy (ΔH) is a measure of heat energy change in a system. If heat is released (exothermic), ΔH is negative, indicating a spontaneous process.
- 😀 Temperature affects spontaneity. If both ΔH and ΔS are positive, the reaction is spontaneous above a certain temperature.
- 😀 Gibbs Free Energy (ΔG) is a key indicator of whether a reaction is spontaneous: ΔG < 0 means spontaneous, ΔG > 0 means non-spontaneous.
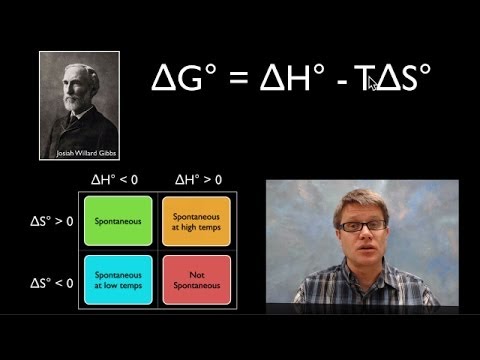
- 😀 ΔG is calculated as ΔG = ΔH - TΔS, where T is temperature. This equation determines whether a process will be spontaneous.
- 😀 If ΔH < 0 and ΔS > 0, ΔG will always be negative, indicating the reaction is spontaneous at all temperatures.
- 😀 If ΔH > 0 and ΔS < 0, ΔG will always be positive, making the reaction non-spontaneous at all temperatures.
- 😀 For reactions where both ΔH and ΔS are positive, spontaneity depends on temperature. At higher temperatures, the reaction can become spontaneous.
- 😀 A reaction with both negative ΔH and negative ΔS may be spontaneous at low temperatures but non-spontaneous at high temperatures.
- 😀 The melting of ice is a good example of an endothermic and spontaneous process. Although ΔH is positive, the process is still spontaneous due to the effect of temperature and entropy.
Q & A
What is entropy, and how does it relate to the disorder of a system?
-Entropy is a measure of the degree of disorder or randomness in a system. For example, when gas particles in a closed container spread out after the lid is removed, the system becomes more disordered, leading to an increase in entropy.
What is the significance of a spontaneous process in relation to entropy?
-A spontaneous process is one that occurs naturally without external influence. If the entropy of a system increases (i.e., becomes positive), the process is considered spontaneous.
How is enthalpy related to spontaneous processes?
-Enthalpy change (ΔH) indicates whether a process is exothermic (releases heat). If ΔH is negative, it typically corresponds to a spontaneous, exothermic process, where heat is released to the surroundings.
How does temperature affect the spontaneity of a process?
-Temperature plays a crucial role in determining whether a process is spontaneous. An increase in temperature can either increase or decrease the rate of a spontaneous process, depending on other factors like enthalpy and entropy.
What is Gibbs free energy, and why is it important in determining spontaneity?
-Gibbs free energy (ΔG) combines the effects of enthalpy and entropy to determine whether a process is spontaneous. If ΔG is negative, the process is spontaneous; if ΔG is positive, the process is non-spontaneous. ΔG provides a more comprehensive understanding of spontaneity than ΔH or ΔS alone.
What does it mean if ΔG is negative, positive, or zero?
-If ΔG is negative, the process is spontaneous. If ΔG is positive, the process is non-spontaneous. If ΔG is zero, the system is at equilibrium, meaning the forward and reverse reactions occur at the same rate.
What happens when ΔH is negative and ΔS is positive?
-When ΔH is negative (exothermic) and ΔS is positive (entropy increases), the process is always spontaneous because ΔG will be negative in all temperature conditions.
How do the signs of ΔH and ΔS affect the spontaneity of a process at different temperatures?
-If both ΔH and ΔS are positive, the spontaneity of the process depends on temperature. The process is spontaneous above a certain temperature where the term -TΔS becomes greater than ΔH. If both ΔH and ΔS are negative, the process is spontaneous at lower temperatures.
What is the condition for a process to be spontaneous above a certain temperature?
-For a process to be spontaneous above a certain temperature, the value of ΔH must be positive, and ΔS must also be positive. This ensures that at high enough temperatures, the -TΔS term dominates, making ΔG negative.
In the example of magnesium oxide reacting with carbon, what is the temperature above which the reaction becomes spontaneous?
-For the reaction between magnesium oxide and carbon, with ΔH = 491.1 kJ/mol and ΔS = 198 J/K·mol, the reaction becomes spontaneous above a temperature of 2480.3 K. Below this temperature, the reaction is non-spontaneous.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة

16.2 Driving Forces of Reactions

Bioquímica - Aula 10 - O sentido das reações metabólicas

Thermodynamics Class 11 in 5 Minutes | Chemistry | Quick Revision | NEET, JEE & CBSE |

004-Energy & Metabolism
Using Gibbs Free Energy

4.CHEMICAL THERMODYNAMICS| EASY TRICK TO LEARN|ONE SHOT | COMPLETE CHAPTER IN 20 MINS|PRADEEP SIR
5.0 / 5 (0 votes)
