GCSE Chemistry - Le Chatelier's Principle #50 (Higher Tier)
Summary
TLDRThis video explains Le Chatelier's Principle, which describes how the position of equilibrium in a reversible reaction shifts in response to changes in temperature, pressure, and concentration. It illustrates this concept through the production of ammonia from nitrogen and hydrogen, highlighting how a decrease in temperature favors the exothermic reaction, while an increase shifts the equilibrium to the left. Changes in pressure and concentration also influence the reaction, with the equilibrium moving towards the side with fewer molecules when pressure is increased and opposing changes in concentration. Understanding these shifts helps predict reaction behavior under varying conditions.
Takeaways
- 😀 Le Chatelier's Principle explains how the position of equilibrium in a reversible reaction is affected by changes in temperature, pressure, and concentration.
- 😀 The position of equilibrium refers to the ratio of reactants to products in a chemical reaction at equilibrium.
- 😀 The equilibrium lies to the left if there are more reactants and to the right if there are more products.
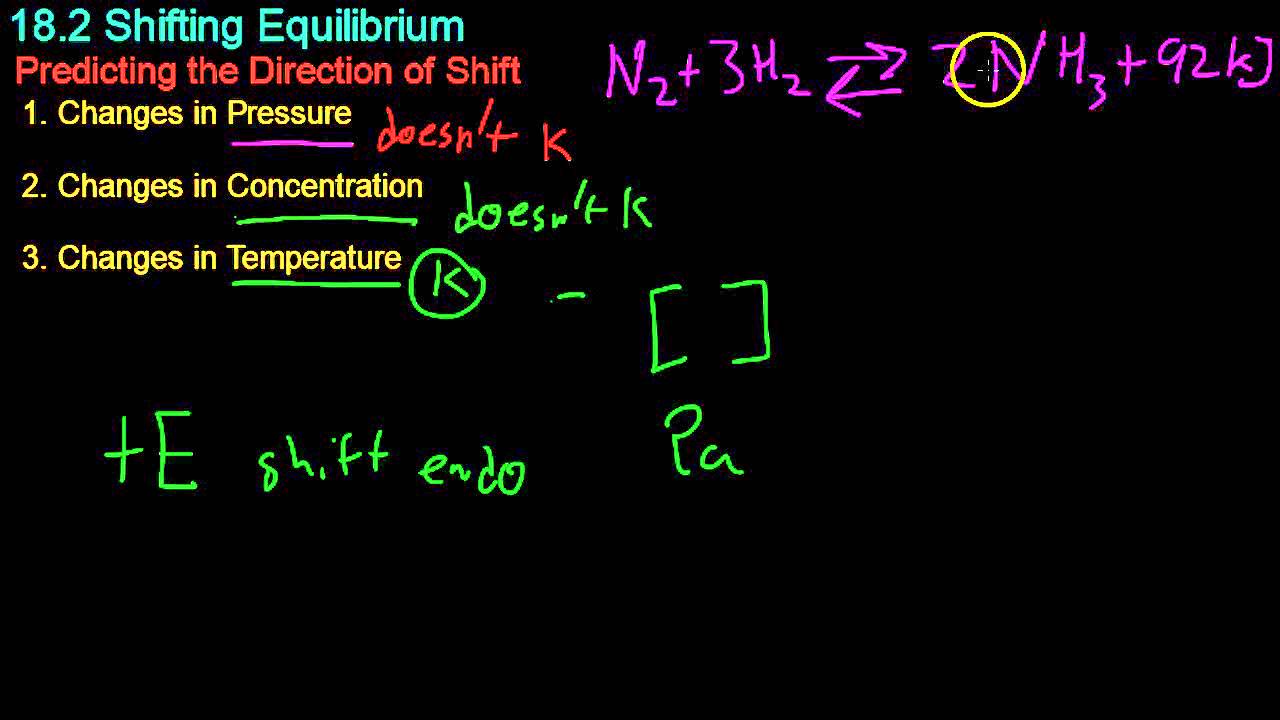
- 😀 If the temperature is decreased in an exothermic reaction, the equilibrium shifts to the right to produce more products.
- 😀 Conversely, increasing the temperature in an exothermic reaction causes the equilibrium to shift to the left, favoring reactants.
- 😀 In a sealed system, increasing pressure will shift the equilibrium to the side with fewer gas molecules to counteract the change.
- 😀 Decreasing pressure will shift the equilibrium to the side with more gas molecules to increase pressure again.
- 😀 Adding more reactants (like nitrogen) will shift the equilibrium to the product side to counteract the increase in concentration.
- 😀 The production of ammonia from nitrogen and hydrogen demonstrates the application of Le Chatelier's Principle.
- 😀 Understanding Le Chatelier's Principle helps predict the behavior of chemical reactions under different conditions.
Q & A
What is Le Chatelier's Principle?
-Le Chatelier's Principle states that if the conditions of a reversible reaction are changed, the position of equilibrium will shift to counteract that change.
How does temperature affect the position of equilibrium?
-Decreasing the temperature of a system will shift the equilibrium in the exothermic direction, while increasing the temperature will shift it in the endothermic direction.
In the ammonia production reaction, what is the overall energy change and what does it indicate?
-The overall energy change is -92 kJ/mol, indicating that the forward reaction (producing ammonia) is exothermic, meaning it releases energy.
What happens to the equilibrium position when pressure is increased in a sealed system?
-Increasing the pressure causes the equilibrium to shift to the side with fewer molecules to reduce the pressure.
Why does the equilibrium shift to the right when the pressure is increased in the ammonia reaction?
-In the ammonia reaction, the right side has two molecules (ammonia) compared to four on the left (one nitrogen and three hydrogens), so the equilibrium shifts to the right to decrease the pressure.
What effect does decreasing the pressure have on the equilibrium position?
-Decreasing the pressure will shift the equilibrium to the side with more molecules in order to increase the pressure again.
How does changing the concentration of reactants affect the equilibrium position?
-If the concentration of a reactant, like nitrogen, is increased, the equilibrium will shift to the opposite side (to the right) to form more products (ammonia).
Can Le Chatelier's Principle be applied to reactions other than the production of ammonia?
-Yes, Le Chatelier's Principle can be applied to any reversible reaction to predict how changes in conditions will affect the position of equilibrium.
What are the three main factors that can affect the position of equilibrium according to the script?
-The three main factors are temperature, pressure, and concentration.
What is the significance of understanding Le Chatelier's Principle in chemical reactions?
-Understanding Le Chatelier's Principle allows chemists to predict how changes in conditions can affect the yield of products in chemical reactions, which is crucial for industrial applications.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة

Deslocamento de Equilíbrio - Princípio de Le Chatelier

11 клас. Хімія. Необоротні та оборотні хімічні реакції. Хімічна рівновага. Принцип Ле Шательє
18.2 Shifting Equilibrium

PERGESERAN KESETIMBANGAN (ASAS LE CHATELIER) : KESETIMBANGAN KIMIA KELAS 11

Kesetimbangan Kimia | Faktor Yang Mempengaruhi Pergeseran Kesetimbangan
Le Chatelier's Principle
5.0 / 5 (0 votes)
