6. Chemical Reactions (Part 3) (3/5) (Cambridge IGCSE Chemistry 0620 for 2023, 2024 & 2025)
Summary
TLDRThis video from IGCC Study Bud covers part three of topic six on chemical reactions, focusing on reversible reactions and equilibrium. It explains the differences between irreversible and reversible reactions, the concept of equilibrium, and the impact of changes in temperature, pressure, and concentration on equilibrium. The video also explores hydrated and anhydrous compounds, highlighting how heat and water affect their reversible reactions. Lastly, it discusses how catalysts speed up reactions without affecting equilibrium. Viewers are encouraged to like, subscribe, and engage in the comment section.
Takeaways
- 🔄 Reversible reactions allow products to revert to reactants, unlike irreversible reactions where the reaction ends.
- ⚖️ Equilibrium is reached in a closed system when the rate of the forward reaction equals the reverse reaction, keeping reactant and product concentrations constant.
- 💧 Hydrated compounds contain water molecules in their structure, while anhydrous compounds do not.
- 🔥 Heating hydrated compounds, like copper sulfate, removes water molecules and turns them anhydrous, changing their color.
- 💦 Adding water to anhydrous compounds reverses the reaction, converting them back to their hydrated forms.
- 🌡️ Changing temperature shifts equilibrium: raising temperature favors the endothermic reaction, while lowering temperature favors the exothermic reaction.
- 💨 In gas reactions, increasing pressure shifts equilibrium towards the side with fewer gas molecules, while decreasing pressure shifts it towards the side with more gas molecules.
- 📊 Changing reactant concentration affects equilibrium: increasing reactants shifts equilibrium toward products, while decreasing reactants shifts it toward reactants.
- ⚗️ Catalysts speed up reactions but do not affect the equilibrium position; they help the system reach equilibrium faster.
- 🎬 The video concludes with a reminder to like, subscribe, and support the channel, and encourages feedback and suggestions.
Q & A
What is a reversible reaction?
-A reversible reaction is one where the products of the reaction can react with each other to form the original reactants. This means the reaction can proceed in both directions, from reactants to products and back again.
What symbol is used to represent a reversible reaction?
-A reversible reaction is represented by a double-headed arrow symbol (⇌), which indicates that the reaction can go in both directions.
What does it mean for a reaction to be in equilibrium?
-Equilibrium refers to a state where the forward and reverse reactions occur at the same rate, meaning the concentrations of reactants and products remain constant over time in a closed system.
What are hydrated compounds?
-Hydrated compounds are substances that have water molecules trapped within their structure.
What are anhydrous compounds?
-Anhydrous compounds are substances that do not contain water molecules within their structure.
How does heating affect hydrated compounds?
-Heating hydrated compounds causes them to lose their water molecules and become anhydrous. For example, heating hydrated copper(II) sulfate changes its color from blue to white as it becomes anhydrous.
How does adding water to anhydrous compounds affect them?
-Adding water to anhydrous compounds can cause them to regain their water molecules and revert to their hydrated form. This change is reversible, as seen with anhydrous copper(II) sulfate turning back to its blue hydrated form when water is added.
What factors affect the position of equilibrium in a reversible reaction?
-The position of equilibrium can be affected by changing conditions such as temperature, pressure (for gaseous reactions), and the concentration of reactants or products.
How does changing temperature affect the position of equilibrium?
-Raising the temperature shifts the equilibrium towards the endothermic reaction to absorb heat, while lowering the temperature shifts it towards the exothermic reaction to release heat.
How does pressure affect equilibrium in gaseous reactions?
-Increasing pressure shifts the equilibrium towards the side of the reaction with fewer gas molecules to reduce pressure. Decreasing pressure shifts it towards the side with more gas molecules to increase pressure.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة

6. Chemical Reactions (Part 2) (2/5) (Cambridge IGCSE Chemistry 0620 for 2023, 2024 & 2025)

EQUILÍBRIO QUÍMICO: DEFINIÇÃO, CÁLCULOS E GRÁFICOS | Resumo de Química para o Enem
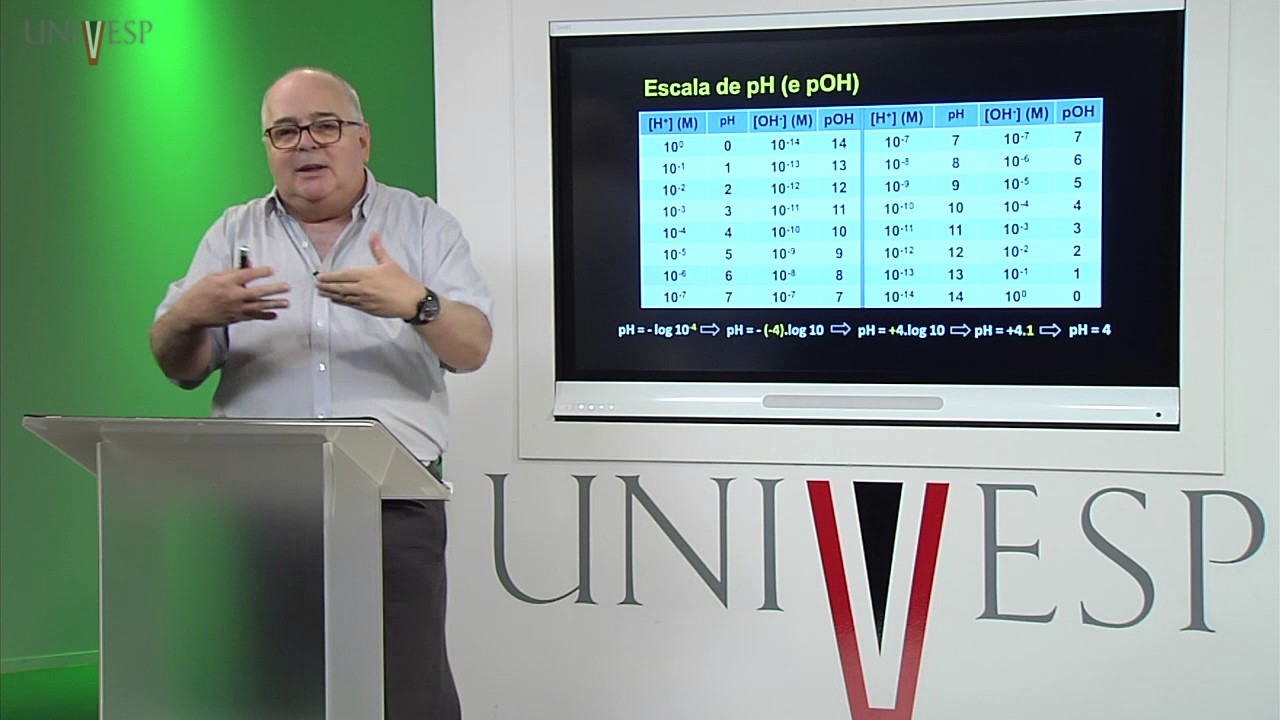
Bioquímica - Aula 03 - Alguns conceitos químicos importantes - 2
Chemical Equilibrium Grade 12 Chemistry

AQA 1.6 Equilibria REVISION

Kesetimbangan Kimia| Kimia SMA | Tetty Afianti
5.0 / 5 (0 votes)
