Particles and waves: The central mystery of quantum mechanics - Chad Orzel
Summary
TLDRThe script explores the dual nature of particles and waves in quantum physics, starting with Einstein's explanation of light as photons with discrete energy. It continues with Rutherford's atomic model and Bohr's quantized orbits, leading to de Broglie's hypothesis of electron wave-particle duality. The script concludes with the wave-particle behavior of electrons, emphasizing its significance as the core mystery of quantum mechanics.
Takeaways
- 🌌 Everything in the universe exhibits both particle and wave characteristics.
- 🔬 The dual nature of light was first suggested by Albert Einstein, building on Max Planck's earlier work.
- 💡 Planck proposed that light is emitted in discrete energy units, a concept Einstein applied to photons.
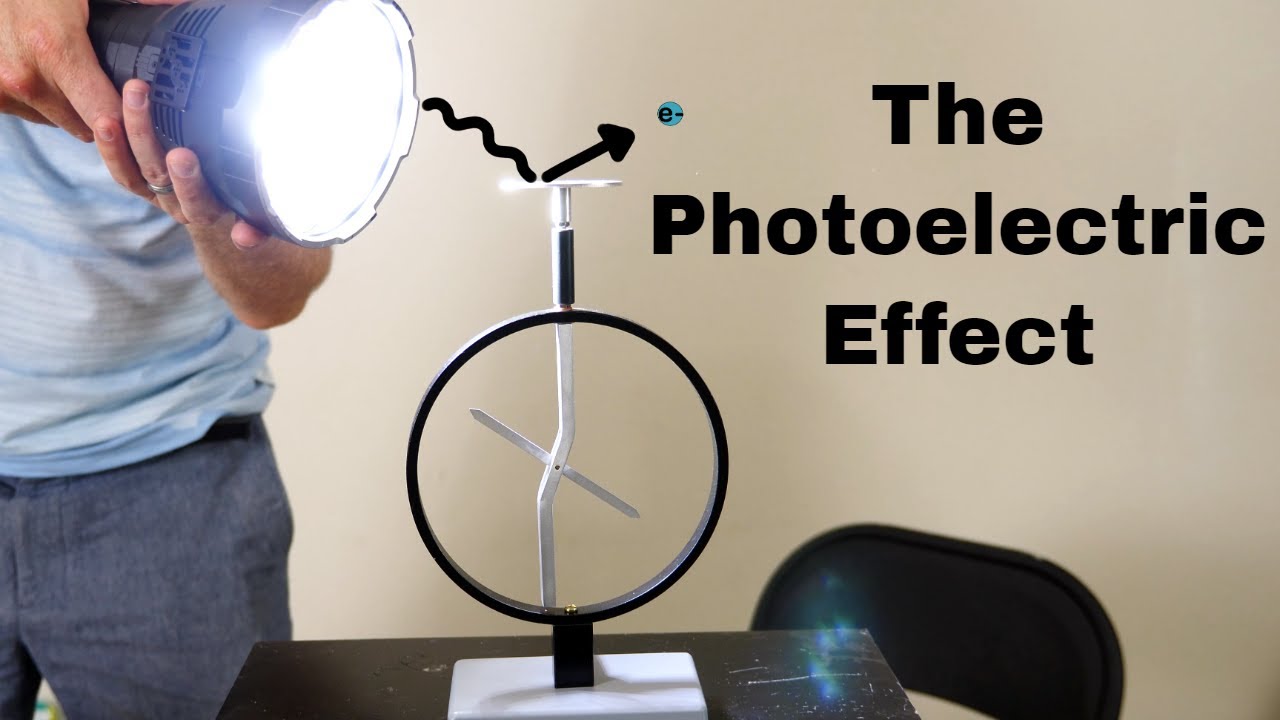
- 🎯 Einstein's photon theory explained the photoelectric effect where light knocks electrons loose from metals.
- 🧲 Ernest Rutherford's experiment showed that atoms have a dense nucleus, leading to the 'solar system' model of the atom.
- ⚛️ Rutherford's model faced the problem of electrons spiraling into the nucleus, which was addressed by Niels Bohr.
- 🌀 Bohr proposed that electrons in certain orbits do not emit light, which explained atomic stability and spectral lines.
- 🔍 Louis de Broglie suggested that if light can act as particles, electrons might also behave as waves.
- 🌊 De Broglie's hypothesis was confirmed when electron wave behavior was observed in double-slit experiments.
- 🧠 The wave-particle duality is central to quantum mechanics, as emphasized by Richard Feynman.
Q & A
What is the dual nature of light?
-The dual nature of light refers to the concept that light behaves both as a particle and a wave.
Who first suggested the dual nature of light?
-Albert Einstein first suggested the dual nature of light in 1905, building on earlier ideas from Max Planck.
What did Max Planck propose about the emission of light by hot objects?
-Planck proposed that light is emitted by hot objects in discrete chunks or units of energy that depend on the frequency of the light.
How did Einstein apply Planck's idea to light?
-Einstein applied Planck's idea by suggesting that light, known as a wave, is actually a stream of photons, each with a discrete amount of energy.
What experiment by Ernest Rutherford led to the discovery of the atomic nucleus?
-Rutherford's experiment involved shooting alpha particles at gold atoms, which led to the discovery that most of an atom's mass is concentrated in a tiny nucleus.
What was the problem with Rutherford's model of the atom?
-The problem was that according to classical physics, electrons in Rutherford's model should emit light and spiral into the nucleus, which contradicts the observed stability of atoms.
How did Niels Bohr resolve the issue with Rutherford's atom model?
-Bohr proposed that electrons in certain special orbits do not emit light, and that atoms only absorb and emit light when electrons change orbits.
What was the limitation of Bohr's model of the atom?
-The limitation was that there was no theoretical reason given for why certain orbits were special.
What contribution did Louis de Broglie make to the understanding of atomic structure?
-De Broglie suggested that if light behaves like a particle, then electrons, which are particles, might also behave like waves.
How does the wave-like behavior of electrons explain Bohr's rule for special orbits?
-The wave-like behavior of electrons allows for the explanation that only certain orbits correspond to stable wave patterns, which is why they are special.
What experiment provides evidence for the wave-particle duality of electrons?
-The double-slit experiment with electrons shows wave-particle duality, as individual electrons behave as particles, but collectively form a wave interference pattern.
Why is the wave-particle duality considered one of the most powerful ideas in physics?
-The wave-particle duality is considered powerful because it forms the basis of quantum mechanics and explains many phenomena that cannot be understood through classical physics alone.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة

Albert Einstein and The Photoelectric Effect | AMS OpenMind

MIT Quantum Experiment Proves Einstein Wrong After 100 years

Photon Energy | Physical Processes | MCAT | Khan Academy

Quantum Mechanics - Part 1: Crash Course Physics #43
Knocking Electrons With Light—The Photoelectric Effect

Konsep Dasar Fisika Modern-Kuantum
5.0 / 5 (0 votes)
