Photon Energy | Physical Processes | MCAT | Khan Academy
Summary
TLDRThis script delves into the dual nature of light, traditionally viewed as a wave with oscillating electric and magnetic fields. It explains how light exhibits wave-like behaviors such as diffraction and interference. However, physicists discovered in the late 1800s and early 1900s that light also displays particle-like properties, termed as quantum mechanics, where light can only deposit energy in discrete amounts. These particles of light are called photons. The script introduces Planck's constant and the formula to calculate the energy of a photon, emphasizing the small scale of this energy which makes light appear continuous to the naked eye but is actually composed of discrete energy packets.
Takeaways
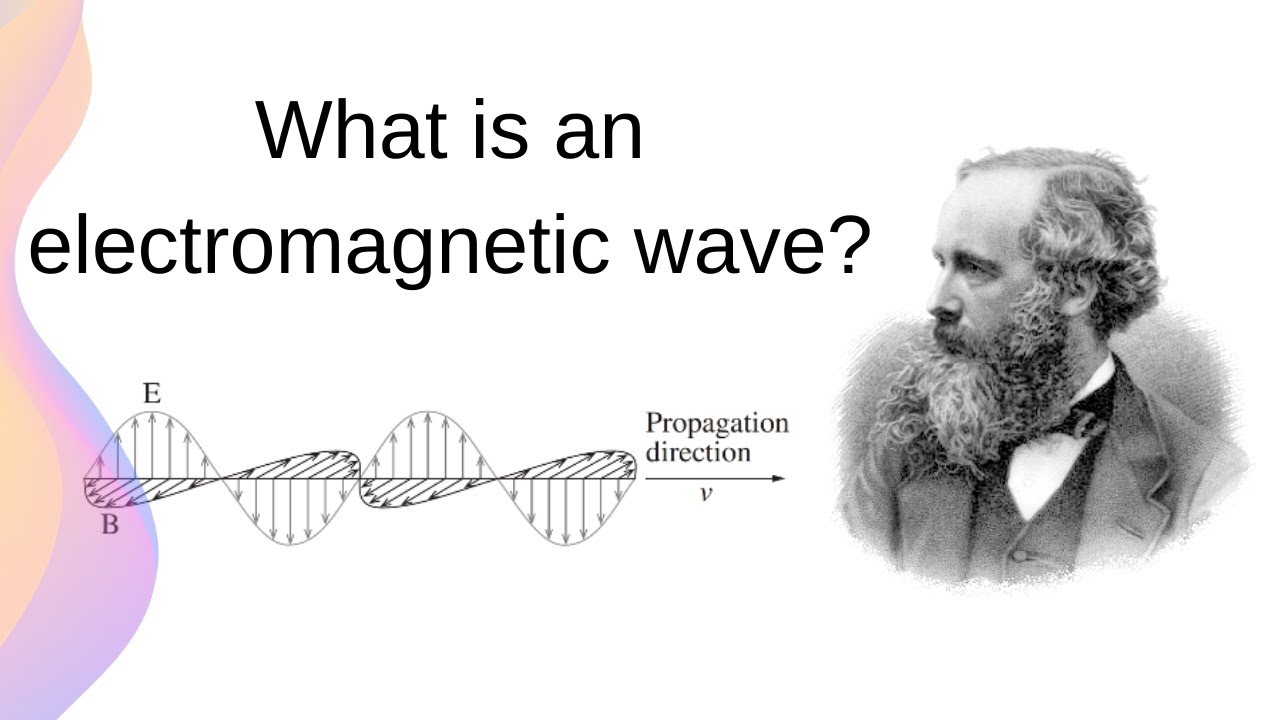
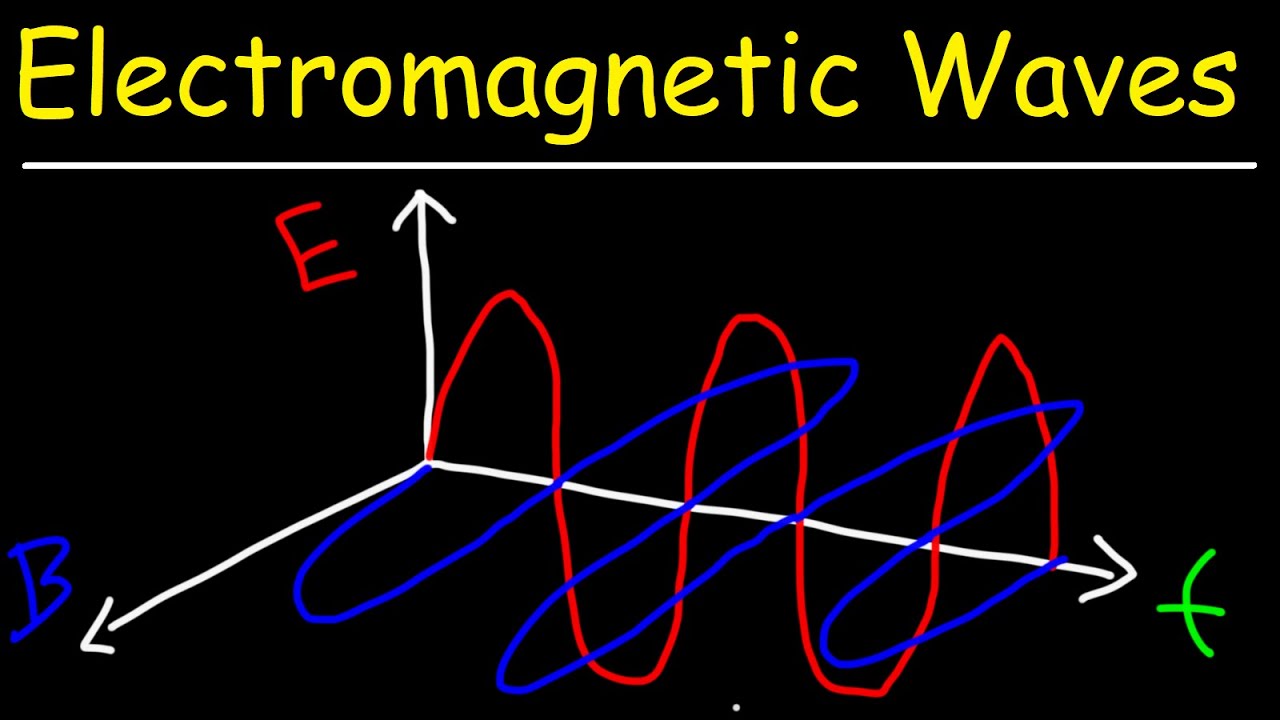
- 🌊 Light has traditionally been treated as a wave with oscillating electric and magnetic fields.
- 🔍 Light exhibits wave-like behaviors such as diffraction and interference.
- 💡 In the late 1800s and early 1900s, physicists discovered that light also displays particle-like behavior.
- 📏 Light can only deposit discrete amounts of energy, known as quanta, which led to the development of quantum mechanics.
- ✨ The particles of light are called photons, which deposit energy in specific, discrete amounts.
- 📐 Planck's constant is crucial in determining the energy of a photon, represented by the formula E = h*f, where h is Planck's constant and f is the frequency.
- 📉 Planck's constant is extremely small (6.626 x 10^-34 joule seconds), making the energy of individual photons very small.
- 🔬 Light energy appears continuous on a macroscopic scale, but at a microscopic level, it is absorbed in discrete chunks.
- ⚛️ The discrete nature of photon absorption means energy is deposited in steps, not continuously, even though it may appear smooth on a larger scale.
- ⚡ The concept of wave-particle duality is central to understanding light and electromagnetic radiation in modern physics.
Q & A
Why was light initially considered a wave?
-Light was initially considered a wave because it exhibits wave-like behaviors such as diffraction and interference when passing through small openings or overlapping with itself.
What discovery challenged the wave theory of light?
-Physicists discovered in the late 1800s and early 1900s that light, and all electromagnetic radiation, can also display particle-like behavior.
What is the term used to describe light's dual wave-particle nature?
-The term used to describe light's dual wave-particle nature is 'wave-particle duality'.
What are the particles of light called?
-The particles of light are called photons.
Why is it difficult to visualize the wave-particle duality of light?
-It is difficult to visualize the wave-particle duality of light because there is no classical analog for something that can exhibit both wave-like and particle-like characteristics, making it hard to draw a visual representation.
What is the significance of the term 'quantum' in quantum mechanics?
-In quantum mechanics, 'quantum' signifies a discrete jump or a chunk of energy that light can deposit, with no less than that amount being possible.
What is Planck's constant and what role does it play in quantum mechanics?
-Planck's constant is a fundamental physical constant that describes the scale of quantum effects. It is used in the formula to calculate the energy of a photon and was the first indication that light can only deposit its energy in discrete amounts.
How is the energy of a photon calculated?
-The energy of a photon is calculated using the formula E = hf, where E is the energy, h is Planck's constant, and f is the frequency of the light.
Why did it take so long for physicists to discover the quantum nature of light?
-It took a long time for physicists to discover the quantum nature of light because Planck's constant is extremely small, making the discrete amounts of energy carried by photons also very small and difficult to detect.
What is an example of how the energy of light appears on a macroscopic scale?
-On a macroscopic scale, the energy of light appears to be continuous, similar to the way water flowing from a sink looks continuous despite being made up of discrete water molecules.
How does the energy absorption pattern of a detector on a microscopic scale differ from the macroscopic scale?
-On a microscopic scale, a detector would show a step pattern of energy absorption, with discrete jumps corresponding to the absorption of individual photons. On a macroscopic scale, these jumps appear as a smooth, continuous increase in energy.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)