PERKEMBANGAN TEORI ATOM | Dalton, Thomson, Rutherford, Bohr, Heisenberg, Erwin Schrodinger,
Summary
TLDRThis video explores the fascinating evolution of atomic theory, starting with ancient ideas from Democritus and Aristotle. It covers key models, from Dalton's idea of indivisible atoms to Thomson's discovery of electrons, Rutherford's nuclear atom model, and Bohr's quantized orbits. The video concludes with modern atomic theory, incorporating wave-particle duality and quantum mechanics, explaining how electrons behave in uncertain paths. This journey highlights scientific advancements that have shaped our understanding of matter, making complex concepts accessible and engaging for viewers.
Takeaways
- 😀 Atomic theory has evolved significantly from ancient times to modern science, with the concept of atoms being central to understanding matter.
- 😀 Democritus proposed that matter is composed of indivisible particles, which he called 'atoms,' laying the groundwork for atomic theory.
- 😀 Aristotle, in contrast to Democritus, believed that matter was continuous and made up of four basic elements: earth, water, fire, and air.
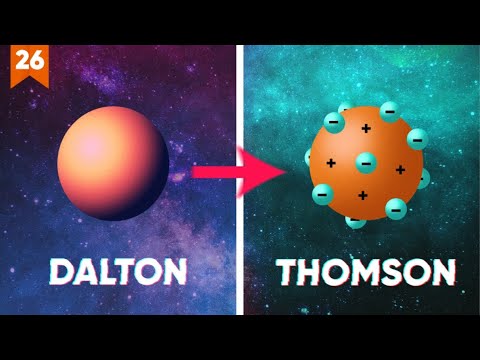
- 😀 John Dalton's atomic model, proposed in 1808, suggested that atoms are indivisible, combine in simple ratios to form compounds, and have distinct properties based on their element.
- 😀 J.J. Thomson's atomic model in 1897 introduced the idea of electrons, particles with negative charge embedded in a positively charged 'pudding' structure.
- 😀 Ernest Rutherford's gold foil experiment in 1910 led to the discovery of the atomic nucleus, which is positively charged and contains most of the atom's mass.
- 😀 Rutherford's model also proposed that electrons orbit the nucleus, but it could not explain why electrons don't spiral into the nucleus due to electrostatic forces.
- 😀 Niels Bohr's atomic model (1913) suggested that electrons travel in fixed orbits around the nucleus without losing energy, and their energy levels are quantized.
- 😀 Despite its success, Bohr's model couldn't explain the spectra of multi-electron atoms or how atoms form molecules through chemical bonds.
- 😀 The modern atomic model, influenced by quantum mechanics, introduced the idea that electrons have dual particle-wave characteristics, and their positions can only be determined probabilistically.
Q & A
What is the role of atoms in our daily life?
-Atoms are the basic building blocks of all matter, present in everything we interact with, from the food we eat to the medicines we take and the computers we use.
How did the concept of atoms evolve over time?
-The idea of atoms has evolved from ancient Greek philosophy to modern scientific discoveries, starting with Democritus's theory of indivisible particles, Aristotelian beliefs in four basic elements, and progressing to scientific models like Dalton's, Thomson's, Rutherford's, Bohr's, and finally modern atomic theory.
What was Democritus's theory about matter?
-Democritus believed that matter is made up of indivisible particles called atoms, and these atoms are in constant motion.
How did Aristotle's view differ from Democritus's?
-Aristotle disagreed with Democritus and argued that matter was continuous and made up of four fundamental elements: earth, water, fire, and air, which could be divided infinitely.
What was Dalton's model of the atom?
-John Dalton's model, proposed in 1808, suggested that atoms are the smallest indivisible particles, each element has atoms of a unique type, atoms combine in simple whole-number ratios to form compounds, and chemical reactions involve the rearrangement of atoms.
What was the flaw in Dalton's atomic model?
-Dalton's model failed to explain that atoms can change into other atoms in nuclear reactions, as it suggested atoms could not be altered through chemical reactions.
What did J.J. Thomson's experiment reveal about atoms?
-J.J. Thomson's cathode ray tube experiment revealed the presence of negatively charged particles, later named electrons, showing that atoms are not indivisible but contain smaller subatomic particles.
What was Rutherford's contribution to atomic theory?
-Ernest Rutherford's gold foil experiment in 1910 led to the discovery of the atomic nucleus, showing that atoms have a small, dense, positively charged nucleus, with electrons surrounding it.
How did Bohr's model improve upon Rutherford's?
-Niels Bohr's model introduced the idea that electrons occupy specific energy levels or orbits around the nucleus, and that they can move between these levels by absorbing or emitting energy.
What is the modern atomic theory, and how does it differ from previous models?
-The modern atomic theory, based on quantum mechanics, explains that electrons behave both as particles and waves. It also states that the position of electrons cannot be precisely determined, only the probability of their location. This theory is a significant departure from earlier models, which treated electrons as particles with fixed positions.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

The Atomic Theory: A Timeline Through History

Quarter 2_WEEK 1 - DAY 1 DALTON AND DEMOCRITUS | Science 8 MATATAG Curriculum

Composition de l'ATOME - Ecriture symbolique | Physique - Chimie

TEORI MODEL ATOM | Fisika Atom #1 - Fisika Kelas 12
O Modelo Atômico de Dalton x Thomson

STRUKTUR ATOM DAN SISTEM PERIODIK UNSUR : Struktur Atom - Materi KIMIA SMA Kelas 10, TKA SMA |Part 1
5.0 / 5 (0 votes)
