BE2103 Thermodynamics in Biosystem_Module 3 Segment 2
Summary
TLDRThis segment of Module 3 in Thermodynamics and Biosystems, presented by Yusuf from the School of Life Sciences, explores energy transfer in systems. It explains how energy crosses system boundaries as heat or work, emphasizing that heat transfer occurs due to temperature differences and stops at thermal equilibrium. The module details three heat transfer mechanisms—conduction, convection, and radiation—and introduces work as energy transferred via force acting over a distance. Key concepts such as adiabatic processes, energy as a path-dependent quantity, and the distinction between state and path functions are discussed, along with conventions for sign and direction in heat and work interactions.
Takeaways
- 🔥 Heat is a form of energy transfer that occurs between two systems due to a temperature difference.
- 🌡️ Energy transfer by heat stops once thermal equilibrium is reached between a body and its surroundings.
- ❄️ Heat transfer into a system is called heat addition, while heat transfer out is called heat rejection.
- 🚫 An adiabatic process is one where no heat transfer occurs, either due to perfect insulation or equal temperatures between the system and surroundings.
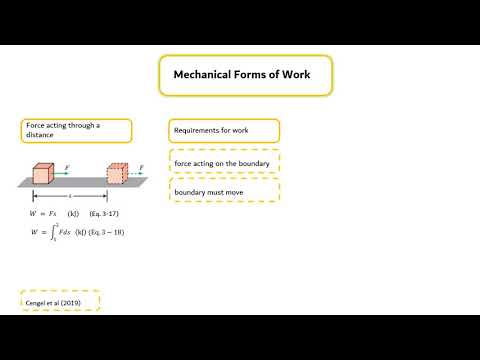
- ⚡ Work is another form of energy transfer, involving a force acting through a distance across the system boundary.
- 📊 Heat and work are path-dependent quantities, meaning their values depend on the process followed, unlike properties which are state functions.
- 🔄 Heat transfer can occur via conduction, convection, or radiation, each involving different mechanisms of energy movement.
- ✅ The direction of heat transfer is always from a higher temperature body to a lower temperature body.
- -
- 📏 The amount of heat transfer can be quantified per unit mass (q) or as a rate per unit time (q̇).
- -
- 💡 Electrical work occurs when electrons move across a potential difference, and mechanical work involves forces such as pistons or shafts acting across boundaries.
- -
- 🧮 Sign conventions are important: heat added to a system and work done by a system are positive; heat leaving a system and work done on a system are negative.
- -
- 🔹 Both heat and work are boundary phenomena—they are recognized only as they cross the system boundary and are associated with processes, not states.
Q & A
What are the two main forms of energy transfer in a closed system?
-The two main forms of energy transfer in a closed system are heat and work.
How is heat defined in thermodynamics?
-Heat is defined as the form of energy transferred between a system and its surroundings due to a temperature difference.
Can heat transfer occur between two systems at the same temperature?
-No, heat transfer cannot occur between two systems at the same temperature because there is no driving force for energy exchange.
What is an adiabatic process?
-An adiabatic process is a process in which no heat is transferred across the system boundary. This can happen if the system is well-insulated or if the system and surroundings are at the same temperature.
What are the three mechanisms by which heat is transferred?
-Heat can be transferred by conduction (through particle interactions), convection (between a solid surface and moving fluid), and radiation (via electromagnetic waves or photons).
How is work defined in thermodynamics?
-Work is defined as energy transfer resulting from a force acting through a distance, such as a moving piston, rotating shaft, or electrical current across a system boundary.
What is the general sign convention for heat and work interactions?
-Heat transfer to a system and work done by a system are considered positive, whereas heat transfer from a system and work done on a system are considered negative.
What is the difference between heat/work and thermodynamic properties?
-Heat and work are path functions, meaning their values depend on the process path, while thermodynamic properties like volume and pressure are point functions, depending only on the system's state.
Why is heat recognized only during transfer and not as a property of a system?
-Heat is recognized only during transfer because it is associated with the process of energy moving across a system boundary, not with the energy contained within the system itself.
How is electrical work calculated in thermodynamic systems?
-Electrical work is calculated based on the movement of electrons through a potential difference in an electric field. The work done is proportional to the number of electrons and the voltage across the system.
What is the difference between point functions and path functions?
-Point functions, such as volume or pressure, depend only on the current state of the system and have exact differentials. Path functions, such as heat and work, depend on the specific process taken to reach a state and do not have exact differentials.
In the context of energy transfer, what does thermal equilibrium mean?
-Thermal equilibrium occurs when a body and its surrounding medium reach the same temperature, at which point heat transfer between them stops.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)