SKL (2)| Kenaikan Titik Didih (∆Tb) | Penurunan Titik Beku (∆Tf)
Summary
TLDRThis educational video explains the colligative properties of solutions, focusing on boiling point elevation (ΔTB) and freezing point depression (ΔTF). The video covers how the addition of solutes affects the boiling and freezing points of solvents, including the differences between non-electrolyte, weak electrolyte, and strong electrolyte solutions. Key formulas are introduced for calculating these changes, along with practical examples like glucose in water. The video also includes phase diagrams to illustrate the effects of solutes on boiling and freezing points, helping viewers understand the underlying chemistry principles.
Takeaways
- 😀 Boiling point (TB) is the temperature at which a liquid's vapor pressure equals the external air pressure, causing it to boil.
- 😀 Freezing point (TF) is the temperature at which a liquid’s vapor pressure matches the solid’s vapor pressure, causing it to freeze.
- 😀 Adding a solute to a solvent increases the boiling point and decreases the freezing point, making the solution harder to boil and freeze.
- 😀 Colligative properties depend on the number of solute particles, not their chemical identity. More solute particles cause higher boiling points and lower freezing points.
- 😀 Solutions are categorized into three types: non-electrolytes (e.g., urea), weak electrolytes (e.g., vinegar), and strong electrolytes (e.g., NaCl).
- 😀 For the same concentration, non-electrolyte solutions have the lowest boiling points and highest freezing points, while strong electrolytes have the highest boiling points and lowest freezing points.
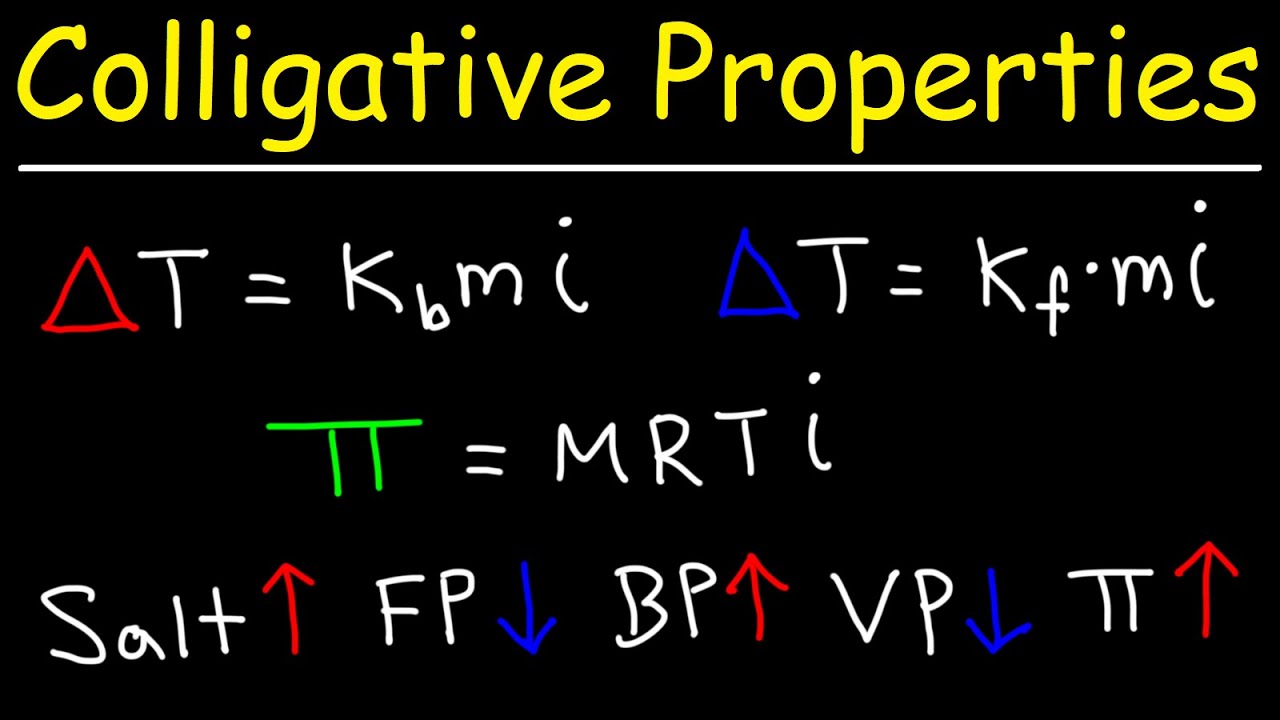
- 😀 Boiling point elevation (ΔTB) and freezing point depression (ΔTF) are calculated using formulas involving the solvent's constants (KB, KF) and molality (m).
- 😀 The equation for non-electrolyte solutions: ΔTB = KB * m, and ΔTF = KF * m.
- 😀 For electrolyte solutions, the equations are adjusted to account for the number of ions (i), using ΔTB = KB * m * i and ΔTF = KF * m * i.
- 😀 An example with glucose dissolved in water demonstrates how to calculate the boiling point elevation and freezing point depression for a non-electrolyte solution, with specific formulas and values for KB and KF.
Q & A
What is the definition of boiling point?
-The boiling point, symbolized as TB, is the temperature at which the vapor pressure of a liquid equals the external air pressure, causing the liquid to start boiling.
How is the freezing point of a substance defined?
-The freezing point, symbolized as TF, is the temperature at which a liquid begins to solidify, or the temperature at which the vapor pressure of the solid equals that of the liquid.
How does adding a solute affect the boiling point of a solvent?
-Adding a solute increases the boiling point of the solvent because the solute makes it harder for the solvent molecules to escape into the vapor phase, requiring a higher temperature to boil.
What is the effect of a solute on the freezing point of a solvent?
-Adding a solute lowers the freezing point of the solvent, meaning the solution freezes at a lower temperature compared to the pure solvent.
What are the three types of solutions based on electrolyte behavior?
-The three types are non-electrolyte solutions (e.g., urea), weak electrolyte solutions (e.g., CH3COOH), and strong electrolyte solutions (e.g., NaCl).
Why do strong electrolyte solutions have a greater effect on colligative properties?
-Strong electrolyte solutions are fully ionized, producing more particles in the solution, which intensifies colligative effects such as boiling point elevation and freezing point depression.
What is the formula for boiling point elevation in a non-electrolyte solution?
-The formula is ΔTB = KB × m, where KB is the boiling point elevation constant and m is the molality of the solution.
How do you calculate the freezing point depression for an electrolyte solution?
-For an electrolyte solution, the freezing point depression is ΔTF = KF × m × i, where KF is the freezing point depression constant, m is the molality, and i is the van’t Hoff factor representing the number of ions produced.
How do you calculate molality (m) of a solution?
-Molality is calculated as m = (mass of solute in grams / molar mass of solute) × (1000 / mass of solvent in grams).
Using the example from the video, what are the boiling point and freezing point of a glucose solution in 200 g of water?
-For 18 g of glucose dissolved in 200 g of water, the boiling point is 100.26°C and the freezing point is -0.93°C.
How can phase diagrams illustrate the changes in boiling and freezing points of a solution?
-Phase diagrams show the temperature and pressure relationships of solid, liquid, and gas phases. When a solute is added, the boiling point curve shifts to higher temperatures and the freezing point curve shifts to lower temperatures, demonstrating boiling point elevation and freezing point depression.
What conclusion can be drawn about acids based on their boiling point and freezing point changes in solutions?
-A weaker acid, like HX in the example, has a lower boiling point and smaller acid constant compared to a stronger acid like HAYE's acid. This is consistent with the relationship between colligative properties and solution strength.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

13.2 Colligative Properties of Solutions (1/2)

Sifat Koligatif Larutan -Kimia SMA kelas 12 semester 1

3 1 1 Penurunan Tekanan Uap, XII MIPA
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure

Simplest Way To Understand Boiling Point Elevation & Vapor Pressure Depression

Química Simples #12 - Resumos - Propriedades Coligativas
5.0 / 5 (0 votes)
