Sulfonation of benzene
Summary
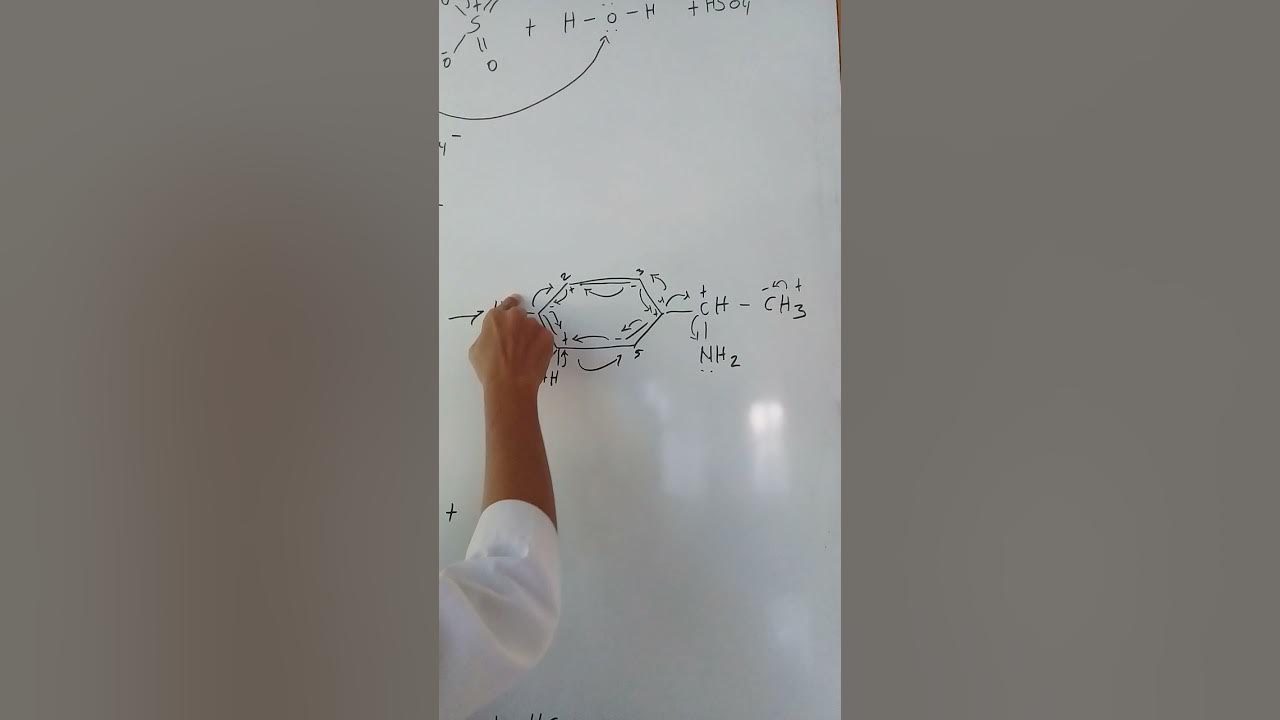
TLDRThis transcript explains the electrophilic aromatic substitution reaction between benzene and sulfur trioxide (SO₃) in the presence of concentrated sulfuric acid, leading to the formation of benzenesulfonic acid. The process involves the protonation of SO₃, which enhances its electrophilic nature. Benzene, despite its stability, undergoes the reaction by attacking the electrophile, breaking its aromatic ring to form a sigma complex carbocation. This complex stabilizes through resonance, and deprotonation occurs to regenerate the aromatic system, resulting in the formation of benzenesulfonic acid. The video highlights how even stable molecules like benzene can react under specific conditions.
Takeaways
- 😀 Benzene undergoes electrophilic aromatic substitution when treated with sulfur trioxide (SO3) and concentrated sulfuric acid.
- 😀 This reaction results in the formation of benzoic sulfonic acid by replacing a hydrogen atom in the benzene ring with the SO3H group.
- 😀 Despite being a stable molecule, benzene reacts under the influence of powerful electrophiles like SO3.
- 😀 SO3 reacts with concentrated sulfuric acid to generate a protonated electrophile that is strong enough to induce a reaction with benzene.
- 😀 The rate-determining step of the reaction involves the formation of a sigma complex, which is a resonantly stabilized carbocation.
- 😀 The carbocation formed in the sigma complex can be stabilized through delocalization of electron deficiency across the aromatic ring.
- 😀 The benzene ring attacks the electrophile, disrupting the aromatic π cloud, leading to the formation of the sigma complex.
- 😀 After the formation of the sigma complex, deprotonation occurs, which restores the aromatic system in the final product.
- 😀 The reaction mechanism involves protonation of sulfur dioxide (SO2) by sulfuric acid, making SO3 even more electrophilic.
- 😀 Ultimately, the aromatic product, benzene sulfonic acid, is regenerated after deprotonation of the sigma complex, demonstrating the electrophilic substitution mechanism.
Q & A
What is the product formed when benzene is treated with sulfur trioxide and concentrated sulfuric acid?
-The product formed is benzoic sulfonic acid.
What type of reaction occurs when benzene reacts with sulfur trioxide and concentrated sulfuric acid?
-This reaction is an electrophilic aromatic substitution.
What is replaced in the benzene ring during the reaction with sulfur trioxide and sulfuric acid?
-A hydrogen atom from the benzene ring is replaced by the SO3H group.
What role does sulfur trioxide (SO3) play in the reaction with benzene?
-Sulfur trioxide is treated with concentrated sulfuric acid to form a protonated electrophile that reacts with the benzene ring.
Why is the formation of the sigma complex the rate-determining step in this reaction?
-The formation of the sigma complex is rate-determining because it involves the benzene ring attacking the electrophile, breaking the aromatic π cloud, and forming a resonantly stabilized carbocation.
What happens to the carbocation formed during the reaction?
-The carbocation formed is delocalized over much of the sigma complex, which helps stabilize it before deprotonation restores the aromaticity of the ring.
How is the electrophile involved in the reaction formed?
-The electrophile is formed when sulfur dioxide (SO2) is protonated by sulfuric acid, making the central sulfur of SO3 more electrophilic.
What is the final product of the reaction in terms of the aromatic system?
-The final product is a benzene sulfonic acid, where the aromatic system is regenerated after deprotonation of the sigma complex.
Why is benzene generally reluctant to react despite being stable?
-Benzene is stable due to its aromaticity, which makes it less reactive. However, it can be induced to react in the presence of powerful electrophiles like SO3 and concentrated sulfuric acid.
What is the significance of the resonance structures in this mechanism?
-The resonance structures help to stabilize the protonated electrophile and the carbocation intermediate, which are crucial for the progression of the electrophilic aromatic substitution reaction.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

Substitusi Elektrofilik Aromatik: Sulfonasi
Proses Sulfonasi Senyawa Aromatik

Kelas XII... Part.3 BENZENA DAN TURUNANNYA

Benzene Reaction | Nitration Reaction: Reaction Mechanism, Nitration Method, Nitration Application

Salt Analysis Anion identification Experiment Edunovus Online Smart Practicals

Electrophilic Aromatic Substitution
5.0 / 5 (0 votes)
