Lesson 5: Biomolecules- Lipids (Part 2)
Summary
TLDRIn this science lesson, students are introduced to lipids, essential biomolecules that store energy for cells. The lesson explains the different types of lipids, including fats, oils, waxes, phospholipids, and steroids, detailing their structures and functions. Students learn about the differences between saturated and unsaturated fats, their sources, and their uses in the body. The role of cholesterol and its importance in hormone production, vitamin D, and cell membranes is also discussed. By the end, students gain a comprehensive understanding of how lipids contribute to energy storage, insulation, and cellular functions.
Takeaways
- 😀 Lipids are biomolecules that store energy in cells, contrasting with carbohydrates that provide instant energy.
- 😀 Lipids are nonpolar and hydrophobic due to their hydrocarbon structure, meaning they are insoluble in water.
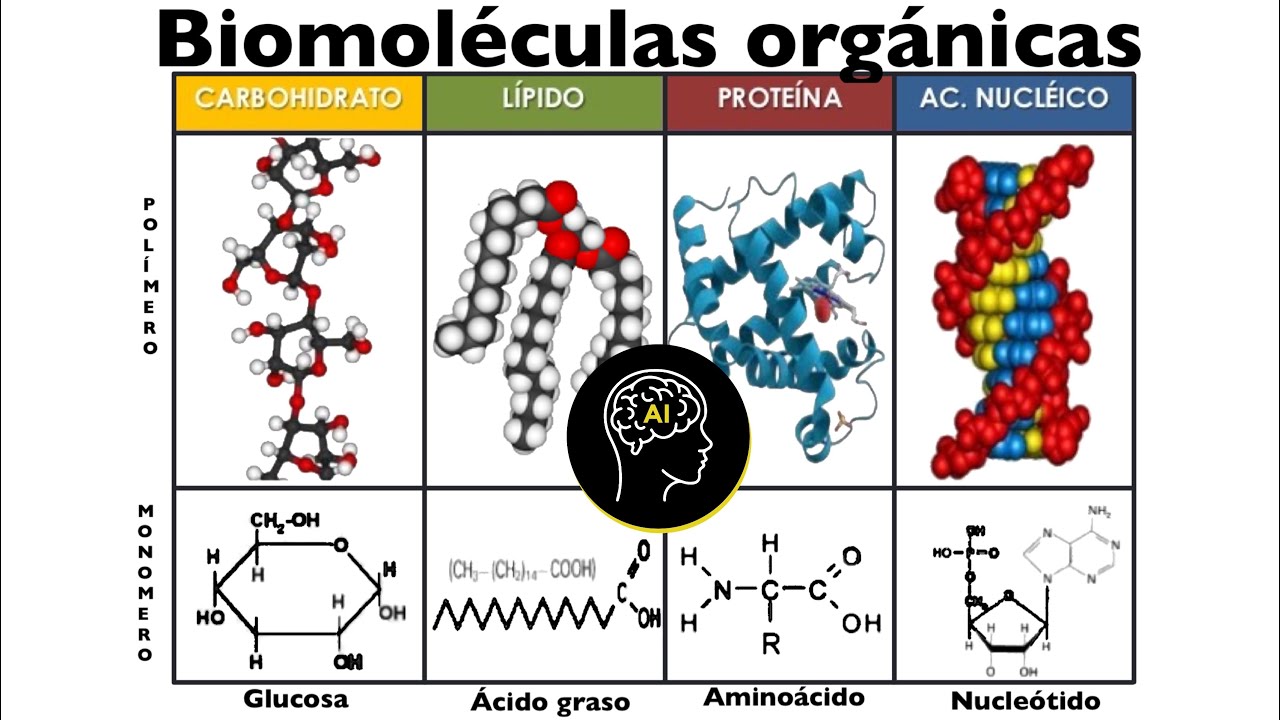
- 😀 Major types of lipids include fats, oils, waxes, phospholipids, and steroids, each serving different functions in cells.
- 😀 Fats and oils consist of glycerol and fatty acids; fats are solid at room temperature, while oils are liquid.
- 😀 Saturated fats have single bonds between carbon atoms and are typically solid at room temperature (e.g., animal fats, butter).
- 😀 Unsaturated fats contain one (monounsaturated) or more (polyunsaturated) double bonds in the carbon chain and are liquid at room temperature (e.g., olive oil, canola oil).
- 😀 Fatty acids, such as stearic acid, palmitic acid, and myristic acid, are found in various animal and plant-based sources.
- 😀 Waxes are lipids that consist of long fatty acid chains attached to alcohols and help prevent water loss on plant and animal surfaces.
- 😀 Phospholipids are amphipathic molecules with both hydrophobic and hydrophilic regions and form the basis of cell membranes.
- 😀 Cholesterol is a steroid that is crucial for various bodily functions, including hormone production, vitamin D synthesis, and bile salt formation.
Q & A
What is the main function of lipids in living organisms?
-The main function of lipids is to store energy for long-term use. They also serve as insulation and are important components of cell membranes and hormones.
What are the two main components of a fat molecule?
-A fat molecule consists of two main components: glycerol and fatty acids.
What is the difference between saturated and unsaturated fats?
-Saturated fats have only single bonds between neighboring carbon atoms in their hydrocarbon chain, while unsaturated fats contain at least one double bond between carbon atoms.
What are triglycerides and how are they formed?
-Triglycerides are formed by the joining of three fatty acids to a glycerol backbone in a dehydration reaction, where three molecules of water are released.
What is the significance of the number of carbons in a fatty acid chain?
-The number of carbons in a fatty acid chain typically ranges from 4 to 36. The most common fatty acids have between 12 to 18 carbons.
How are monounsaturated and polyunsaturated fats different?
-Monounsaturated fats have one double bond in the fatty acid chain, while polyunsaturated fats have multiple double bonds.
What are the sources of saturated fats in the human diet?
-Common sources of saturated fats include fatty meats, animal fats, tallow, cheese, butter, cream, coconut oil, palm oil, and cocoa butter.
What are phospholipids, and why are they important for cells?
-Phospholipids are molecules with two fatty acids and a phosphate group attached to a glycerol backbone. They are essential for the structure of cell membranes, which protect the cell and regulate the movement of substances in and out.
What is cholesterol, and what role does it play in the body?
-Cholesterol is a type of steroid and is essential for the body. It is a precursor to many steroid hormones, vitamin D, and bile salts, and it is also a component of cell membranes.
Why are lipids considered hydrophobic?
-Lipids are considered hydrophobic because they are primarily composed of nonpolar hydrocarbon chains, which do not interact with water, making them insoluble in water.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频
5.0 / 5 (0 votes)