Fluid Mechanics 1.2 - Important Fluid Properties
Summary
TLDRThis lecture delves into foundational concepts in fluid mechanics, including velocity, density, specific weight, specific gravity, and pressure. It distinguishes between liquids and gases in terms of density, explaining how gases have variable density depending on pressure and temperature, while liquids have constant density. The lecture also covers absolute and gauge pressures, explaining their differences and when each is used. Additionally, the speaker highlights the importance of both SI and British gravitational units in engineering, stressing the relevance of understanding both measurement systems for practical applications in the field.
Takeaways
- 😀 Liquids have constant density, meaning their volume cannot be reduced further. For example, one liter of engine oil cannot be squeezed into a smaller volume.
- 😀 Gases, on the other hand, have variable density, and their volume can change depending on pressure and temperature.
- 😀 Specific weight (gamma, γ) is calculated by multiplying density (ρ) by gravity (g). Gravity is 9.81 m/s² in SI units and 32.2 ft/s² in British gravitational units.
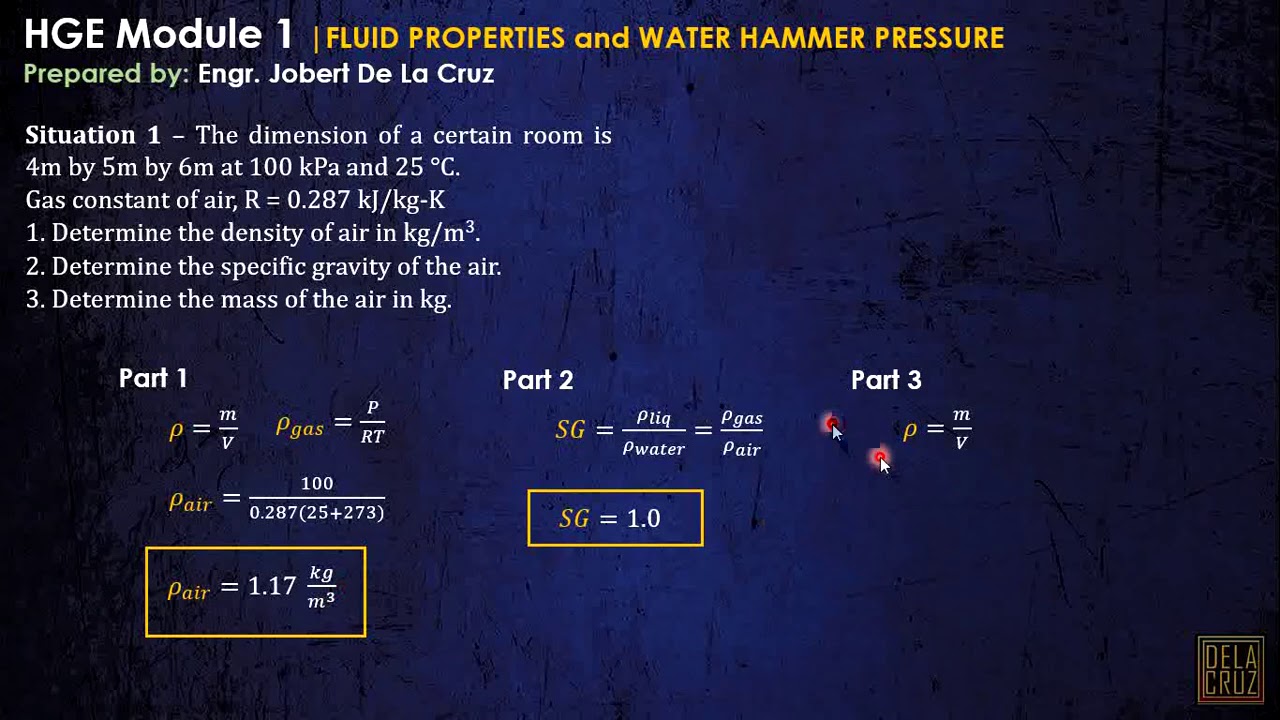
- 😀 Specific gravity is a dimensionless quantity that compares the density of a substance to the density of water at 4°C (999 kg/m³).
- 😀 Mercury's density is approximately 13,600 kg/m³, and its specific gravity is around 13.6, making it easier to refer to than its actual density value.
- 😀 Pressure (P) is the force per unit area. However, pressure calculations should only include the normal force component relative to the surface.
- 😀 Absolute pressure (P_abs) is measured from zero pressure, or vacuum, while gauge pressure (P_g) is measured from atmospheric pressure.
- 😀 Absolute pressure is the total pressure, whereas gauge pressure is the difference between absolute pressure and atmospheric pressure.
- 😀 Absolute pressure cannot be negative, but gauge pressure can be negative (e.g., in the case of a vacuum).
- 😀 In practice, it's crucial to understand both SI (metric) and British gravitational units, as both are used frequently in engineering and industry.
Q & A
What is the relationship between density and volume for liquids and gases?
-For liquids, density is constant because the volume is fixed, meaning you cannot compress liquids further. However, for gases, density is variable, as the volume can change depending on pressure and temperature.
What is specific weight, and how is it calculated?
-Specific weight (gamma) is calculated as the product of density and acceleration due to gravity (gamma = density * g). For SI units, g is 9.81 m/s², and for British gravitational units, it is 32.2 ft/s².
What is specific gravity, and why is it useful?
-Specific gravity is the normalized density of a substance compared to the density of water at 4°C. It is unitless and helps simplify the comparison of densities, especially when using different measurement systems.
Why is specific gravity preferred over using absolute density in some cases?
-Specific gravity is preferred because it simplifies calculations and avoids the need to reference units. For instance, the density of mercury is approximately 13,600, but its specific gravity is about 13.6, making it easier to work with.
What is the definition of pressure?
-Pressure is defined as force per unit area. Specifically, it is the normal force component per area that creates the pressure on a surface.
What is the difference between absolute pressure and gauge pressure?
-Absolute pressure (P_abs) is measured relative to a perfect vacuum (zero pressure), while gauge pressure (P_g) is measured relative to atmospheric pressure. This means gauge pressure can be negative if the pressure is below atmospheric pressure.
Why is the concept of gauge pressure important in real-life applications?
-Gauge pressure is commonly used because it simplifies calculations, especially when atmospheric pressure is treated as the reference point, allowing engineers to work with pressures relative to the atmosphere.
Can pressure ever be negative?
-In the case of absolute pressure, it cannot be negative, as it cannot go below zero pressure (vacuum). However, for gauge pressure, it is possible to have negative values, as it can indicate a pressure below atmospheric pressure.
What is the significance of the units psi and psia in British gravitational systems?
-Psi (pounds per square inch) is used for gauge pressure, while psia (pounds per square inch absolute) is used for absolute pressure. The addition of 'a' in psia helps distinguish between the two types of pressure measurements.
Why is it necessary to understand both SI and British gravitational units in engineering?
-It is important to understand both unit systems because different industries and regions use different systems. For example, British gravitational units are often used in everyday contexts, like in stores, while SI units are more common in scientific and technical fields.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)