BENZENA PART 1 : STRUKTUR DAN TATA NAMA BENZENA
Summary
TLDRThe video explores the structure and applications of benzene and its derivatives, highlighting their significance in everyday products like pain relievers and detergents. It discusses the unique properties of benzene, including its resonance structure and nomenclature rules for naming derivatives. The video details how to identify and name various substituents, emphasizing the importance of priority in naming compounds with multiple substituents. By providing historical context and clear examples, it aims to enhance understanding of organic chemistry concepts related to benzene.
Takeaways
- 😀 Benzene is a compound derived from hydrocarbons, first synthesized by Michael Faraday in 1825.
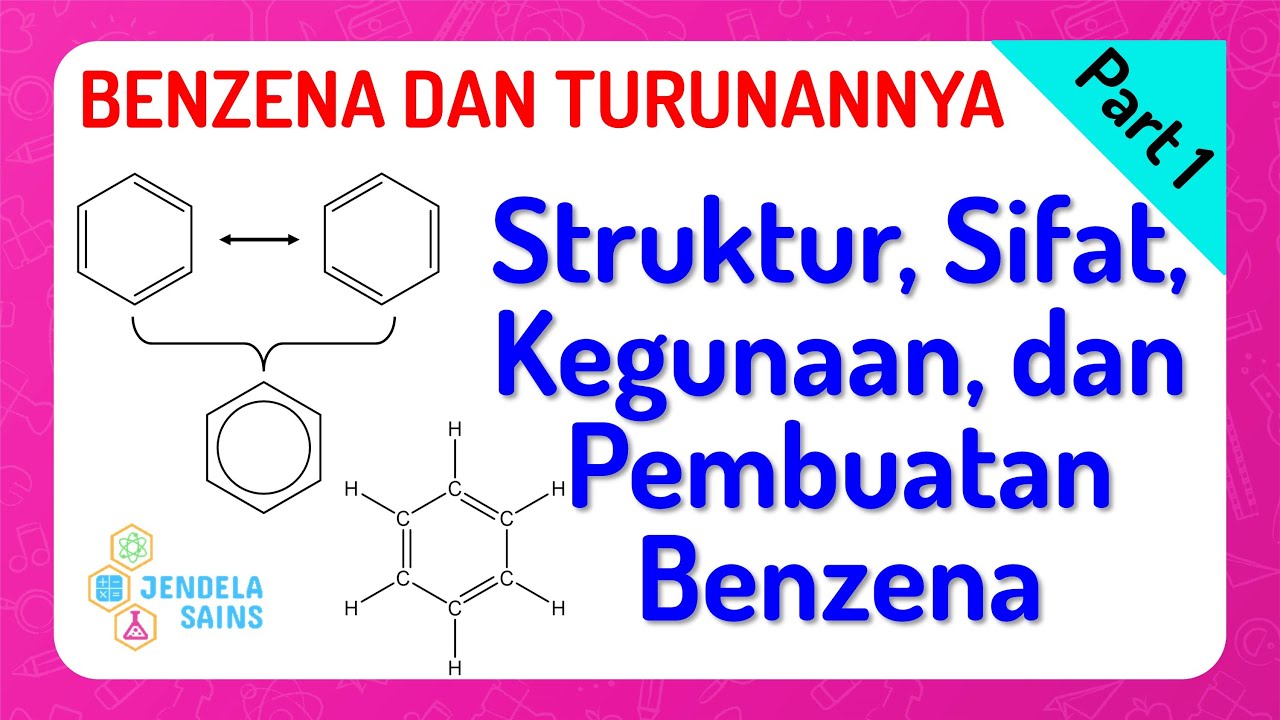
- 😀 The molecular formula of benzene is C6H6, indicating its unique structure with alternating double and single bonds.
- 😀 Benzene's resonance structure allows for delocalized electrons, making it less reactive than alkenes.
- 😀 Substituents in benzene compounds replace hydrogen atoms, leading to various derivative names based on IUPAC and trivial naming conventions.
- 😀 Common benzene derivatives include toluene (methylbenzene), phenol (hydroxybenzene), and aniline (aminobenzene).
- 😀 The naming of disubstituted benzene compounds uses prefixes like ortho, meta, and para to indicate the position of substituents.
- 😀 The priority of substituents is crucial in naming compounds, with functional groups like -OH and -NH2 generally taking precedence.
- 😀 For compounds with three or more substituents, numerical designations are used instead of ortho, meta, and para.
- 😀 Benzene can act as a substituent in larger alkane chains, referred to as phenyl when it is part of a larger structure.
- 😀 Polycyclic aromatic hydrocarbons, such as naphthalene and anthracene, are formed by the fusion of benzene rings.
Q & A
What is benzene and its molecular formula?
-Benzene is a chemical compound with the molecular formula C6H6, consisting of six carbon atoms arranged in a cyclic structure with alternating single and double bonds.
Who first synthesized benzene and in what year?
-Benzene was first synthesized by Michael Faraday in 1825 from gas used for lighting.
What distinguishes benzene's double bonds from those in alkenes?
-Benzene's double bonds are delocalized and do not react in the same way as the localized double bonds in alkenes, which can undergo addition reactions.
What theory explains the stability of benzene's structure?
-The theory of resonance explains the stability of benzene, indicating that its double bonds are delocalized across the ring, forming a stable cyclic structure.
How are benzene derivatives named when they contain substituents?
-Benzene derivatives are named based on the substituents replacing hydrogen atoms, following the IUPAC system, which prioritizes the most significant substituent as the base name.
What is the difference between trivial and IUPAC names for benzene derivatives?
-Trivial names are common names used for certain compounds, while IUPAC names follow a systematic approach based on the structure and substituents of the compound.
How do you determine the priority of substituents in benzene derivatives?
-The priority of substituents is based on their functional groups, with certain groups being ranked higher than others, influencing which is used as the main part of the name.
What are ortho, meta, and para in relation to benzene derivatives?
-Ortho (o), meta (m), and para (p) are terms used to describe the relative positions of substituents on the benzene ring: ortho refers to adjacent positions, meta to one carbon apart, and para to opposite sides.
What is a polycyclic aromatic hydrocarbon?
-Polycyclic aromatic hydrocarbons (PAHs) are compounds made up of multiple fused benzene rings, such as naphthalene and anthracene.
Why is benzene less reactive than alkenes?
-Benzene is less reactive than alkenes due to its stable resonance structure, which makes it less likely to undergo addition reactions compared to the more reactive double bonds of alkenes.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频
Benzena dan Turunannya • Part 1: Struktur, Sifat, Kegunaan, dan Pembuatan Benzena

Benzena dan Turunannya • Part 6: Sifat dan Kegunaan Senyawa Turunan Benzena (2)

BENZENA DAN TURUNANNYA

Benzena dan Turunannya • Part 4: Reaksi Adisi dan Substitusi Benzena

DERIVATIVES in Stock Market - Explained | Mission Options E01

Lecture 27.3 Detergents
5.0 / 5 (0 votes)
