BTEC Applied Science: Unit 5 Physics The First Law of Thermodynamics
Summary
TLDRThis video explains the first law of thermodynamics, detailing how a gas's internal energy is influenced by heat transfer and work done. Internal energy, represented as 'U,' is the sum of the kinetic energies of gas particles. The gas can gain or lose internal energy through heat (Q) or work (W), described by the equation Q = ΔU + W. The video highlights practical examples, such as air in a bicycle pump heating up during compression and the cooling effect when a fridge operates, emphasizing the conservation of energy in thermodynamic processes.
Takeaways
- 😀 The first law of thermodynamics explains the relationship between heat, internal energy, and work.
- 🔥 Internal energy (U) of a gas is the sum of the kinetic energies of its particles.
- 💡 Internal energy can change in two ways: by gaining or losing heat (Q) and through work (W) done by or on the gas.
- 🌡️ When heat is added to a gas, its internal energy and temperature increase.
- ❄️ If a gas loses heat, its internal energy and temperature decrease.
- ⚙️ Work can be done by a gas when it expands, leading to a decrease in internal energy.
- 🔧 Alternatively, when work is done on a gas (e.g., compression), its internal energy increases.
- 📈 The equation Q = ΔU + W represents the conservation of energy in thermodynamics.
- 🚫 In adiabatic processes, no heat is exchanged (Q = 0), and the equation simplifies to ΔU = -W.
- 🤔 Understanding the terms in the equation, like ΔU (change in internal energy), is crucial for grasping thermodynamics.
Q & A
What is the first law of thermodynamics?
-The first law of thermodynamics states that the change in internal energy of a gas is equal to the heat added to the gas minus the work done by the gas.
What does internal energy (U) represent in a gas?
-Internal energy (U) represents the total thermal energy of a gas, calculated as the sum of the kinetic energies of all the gas particles.
How does a gas's internal energy change when it gains heat?
-When a gas gains heat, its internal energy increases, leading to a higher temperature and faster movement of its particles.
What happens to a gas's internal energy when it loses heat?
-When a gas loses heat, its internal energy decreases, resulting in a lower temperature and slower movement of its particles.
What are the two ways a gas's internal energy can change?
-A gas's internal energy can change by gaining or losing heat (Q) or by doing work (W) or having work done on it.
How does work done on a gas affect its internal energy?
-When work is done on a gas, its internal energy increases, often causing the gas to heat up.
What is the relationship between work done by a gas and its internal energy?
-When a gas does work, its internal energy decreases, as the energy is used to perform work rather than contribute to internal energy.
What does the equation Q = ΔU + W represent?
-The equation Q = ΔU + W represents the first law of thermodynamics, indicating that the heat added to the system (Q) is equal to the change in internal energy (ΔU) plus the work done by the system (W).
What happens in an adiabatic process?
-In an adiabatic process, no heat is exchanged with the surroundings (Q = 0), so the relationship simplifies to ΔU = -W, meaning any work done by the gas results in a decrease in internal energy.
Why does the air in a bicycle pump get hotter when compressed?
-The air in a bicycle pump gets hotter when compressed because work is done on the gas, increasing its internal energy.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

Panas Ch. Hukum I Termodinamika - Pembimbing Akademik

Specific Heat Capacity

The First Law of Thermodynamics: Internal Energy, Heat, and Work
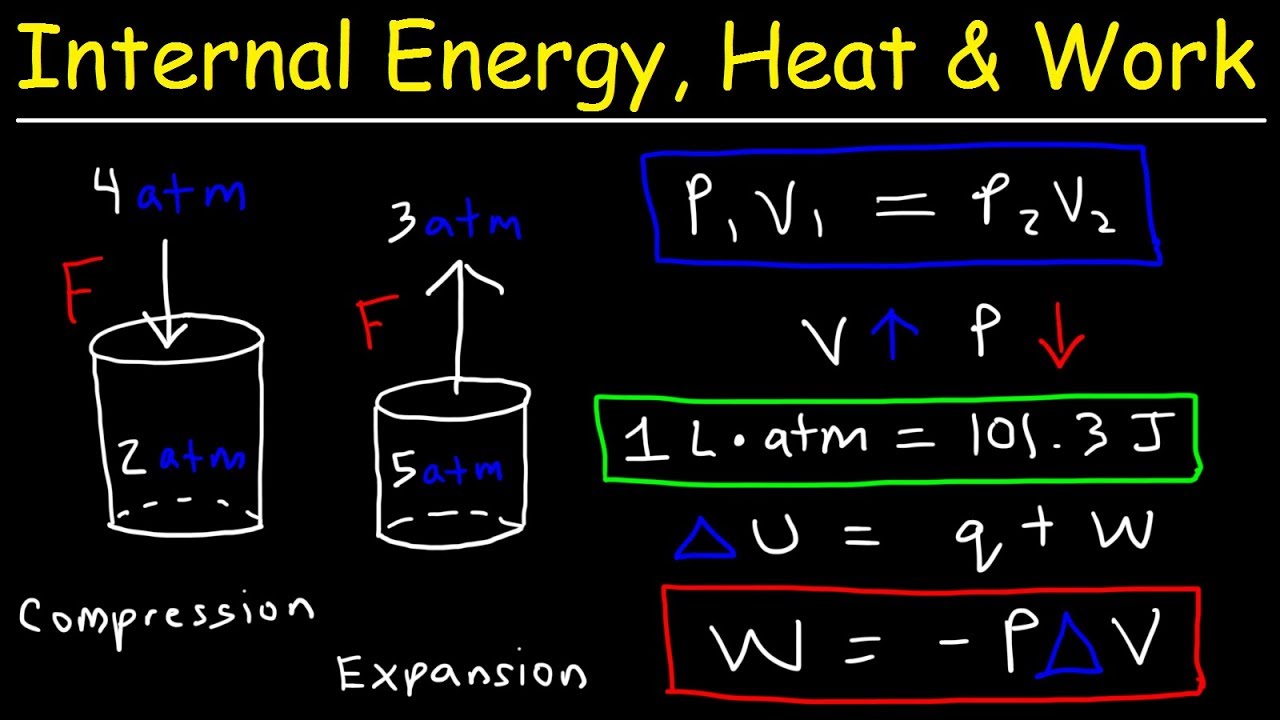
Internal Energy, Heat, and Work Thermodynamics, Pressure & Volume, Chemistry Problems

Isochoric Process Animation

Termodinamika • Part 1: Hukum I Termodinamika Isobarik Isokhorik Isotermik Adiabatik
5.0 / 5 (0 votes)
