Gas Ideal dan Gas Nyata - Kimia Fisika
Summary
TLDRThis lecture introduces key concepts in physical chemistry, focusing on the properties and behavior of gases. It covers the fundamental differences between solids, liquids, and gases, as well as gas laws such as Boyle's, Charles', and Avogadro's. The instructor explains the ideal gas law (PV = nRT), kinetic theory of gases, and the deviations of real gases from ideal behavior. The discussion also explores gas compressibility and concepts like vapor pressure and partial pressure. Overall, the lecture emphasizes the transformation of energy during phase changes and how temperature and pressure affect gas properties.
Takeaways
- 📘 The session is an introduction to physical chemistry, focusing on the chemical and physical properties of matter.
- 🌡️ Matter can change its state due to factors like temperature or pressure, and particles like atoms, molecules, or ions are constantly in motion due to kinetic energy.
- 📏 Solid, liquid, and gas states differ in characteristics such as shape, volume, and flowability, with solids having fixed shapes and volumes, liquids adapting to container shapes, and gases filling the entire container.
- ⚙️ The ideal gas law (PV = nRT) and its related laws (Boyle’s, Charles’, and Gay-Lussac’s) describe the behavior of gases under different conditions of pressure, volume, and temperature.
- 📚 Boyle’s law states that pressure and volume are inversely proportional, while Charles’ law shows that volume and temperature are directly proportional at constant pressure.
- ⚖️ Avogadro’s law indicates that equal volumes of gases at the same temperature and pressure contain the same number of molecules.
- 🔬 Ideal gases follow the gas laws perfectly, but real gases only adhere to these laws at low pressures and high temperatures due to molecular interactions.
- 📊 The compressibility factor (Z) helps determine whether a gas behaves ideally (Z = 1) or deviates from ideal behavior.
- 💨 Real gases exhibit intermolecular forces, which become significant at high pressures or low volumes, leading to phenomena like condensation.
- 🧪 Van der Waals’ equation accounts for the deviations of real gases from ideal behavior by incorporating corrections for intermolecular forces and the volume of gas molecules.
Q & A
What is the subject of the lecture introduced in the transcript?
-The lecture covers the topic of physical chemistry, specifically focusing on the behavior of gases, their properties, and related concepts such as ideal and non-ideal gases.
What are the basic differences between solids, liquids, and gases mentioned in the lecture?
-Solids have a fixed shape and volume, liquids adjust their shape based on the container but have a fixed volume, and gases adjust both their shape and volume based on the container. Additionally, solids are rigid, liquids flow but are less compressible, and gases are highly compressible and fluid.
What is the definition of an ideal gas provided in the transcript?
-An ideal gas is a hypothetical gas that perfectly follows the gas laws, specifically Boyle's, Charles's, and Avogadro's laws, without deviations at any temperature and pressure.
How is the kinetic energy of gas molecules related to temperature?
-The kinetic energy of gas molecules increases with temperature. As temperature rises, the molecules move faster due to the increase in kinetic energy.
What is Boyle's Law as described in the lecture?
-Boyle's Law states that for a fixed amount of gas at constant temperature, the pressure of the gas is inversely proportional to its volume. This means if pressure increases, volume decreases, and vice versa.
What is Charles's Law according to the lecture?
-Charles's Law states that for a fixed amount of gas at constant pressure, the volume of the gas is directly proportional to its temperature. As temperature increases, the volume of the gas also increases.
What does Avogadro's Law explain about gases?
-Avogadro's Law explains that equal volumes of gases at the same temperature and pressure contain an equal number of molecules, regardless of the type of gas.
What is the Ideal Gas Law, and what variables does it connect?
-The Ideal Gas Law is expressed as PV = nRT, connecting pressure (P), volume (V), number of moles (n), gas constant (R), and temperature (T). It describes the state of an ideal gas.
How do real gases deviate from ideal gases according to the lecture?
-Real gases deviate from ideal behavior at high pressures and low temperatures due to intermolecular forces and the finite volume of gas molecules, which are not considered in the ideal gas model.
What is the significance of the Van der Waals equation in relation to real gases?
-The Van der Waals equation modifies the Ideal Gas Law by accounting for the volume of gas molecules and intermolecular forces, providing a more accurate description of the behavior of real gases, especially under conditions of high pressure and low temperature.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

01 02PP01 1StudyofMatter
Key Stage 3 Science (Chemistry) - Properties of Solids, Liquids and Gases

Materi 4 Topik Aliran Kompresibel

Gases: conceitos importantes e características gerais - Brasil Escola
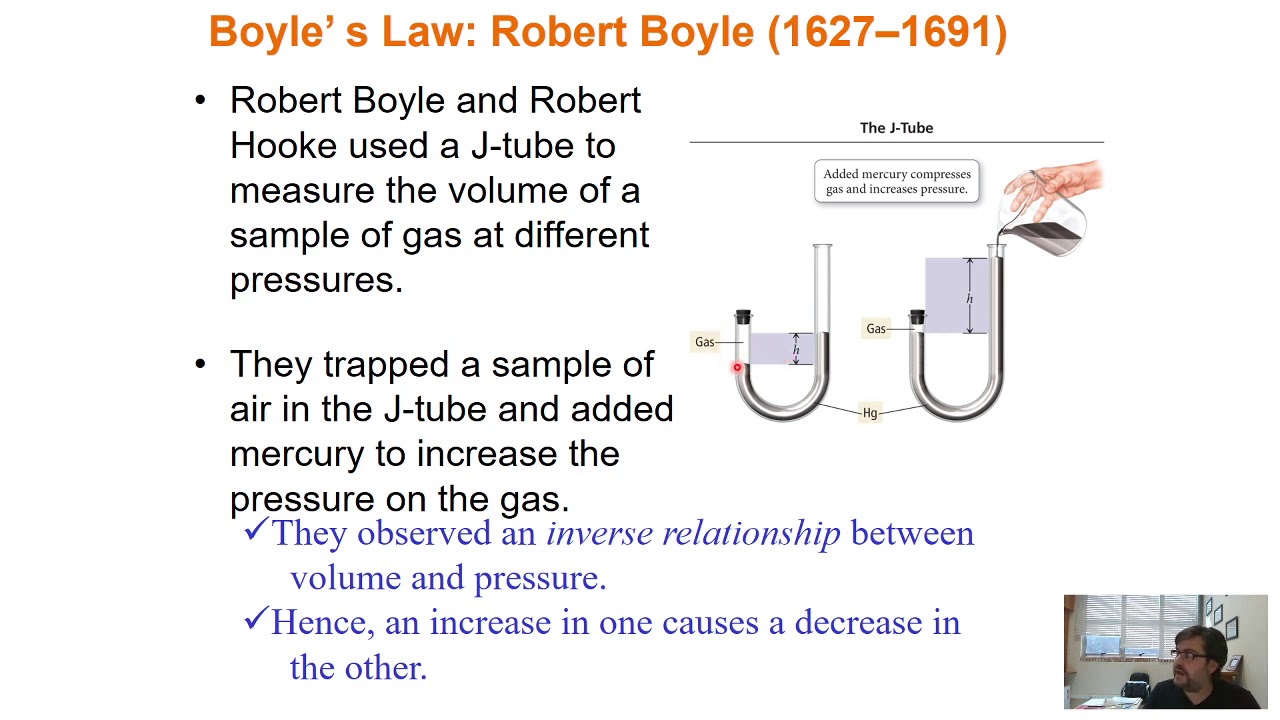
Curso de Físico-Química - Gases Parte 1: Idealidade, Pressão, Lei de Boyle

Mekanika Fluida dan Sifat-sifat Fluida #1
5.0 / 5 (0 votes)
