ATP: Adenosine triphosphate | Energy and enzymes | Biology | Khan Academy
Summary
TLDRIn this video, Sal explains why ATP, or adenosine triphosphate, is considered the energy currency of biological systems. He breaks down the molecular structure of ATP, highlighting the adenosine and three phosphate groups. Sal illustrates how ATP stores energy in its high-energy bonds, which, when broken through hydrolysis, release energy that cells use for various biological processes. He also contrasts ATP with ADP (adenosine diphosphate) and discusses how energy is both stored and released during ATP-ADP conversion, emphasizing ATP's critical role in processes like photosynthesis and cellular function.
Takeaways
- 🔋 ATP (Adenosine Triphosphate) is referred to as the 'currency of energy' in biological systems.
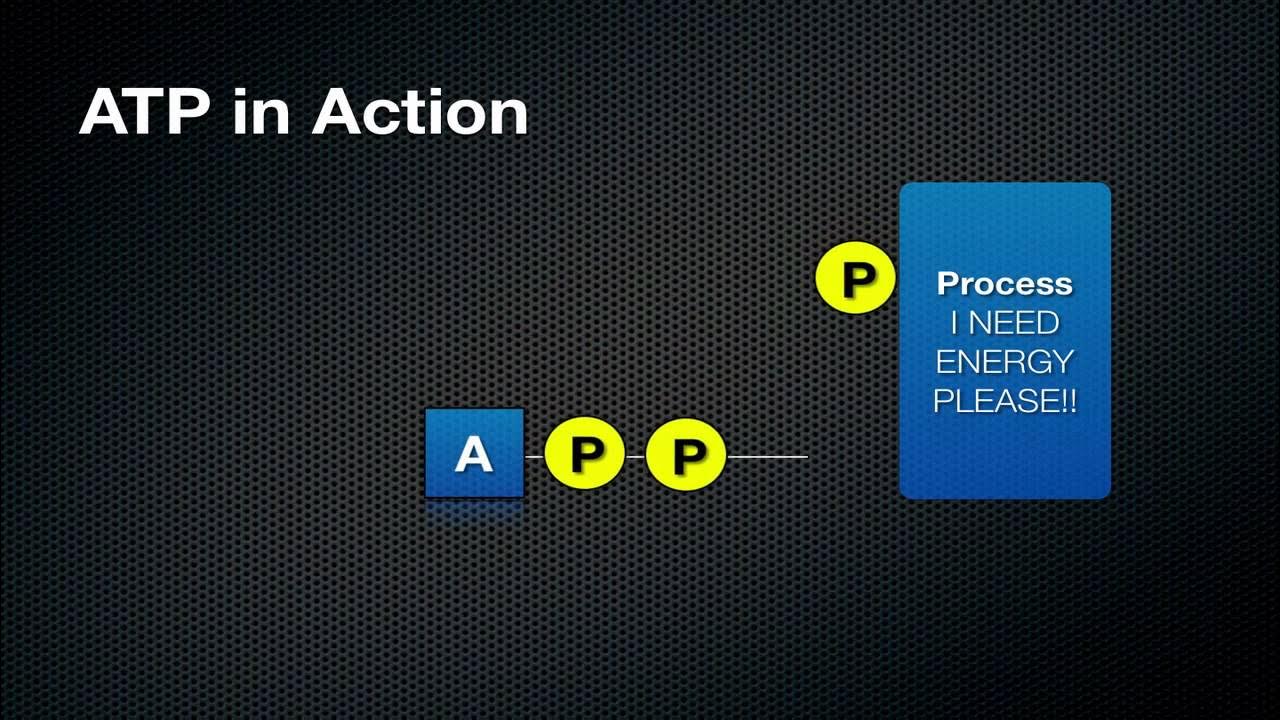
- 🧬 ATP consists of two main parts: adenosine (adenine + ribose) and three phosphoryl groups (triphosphate).
- ⚡ The bonds between the phosphoryl groups are high-energy bonds, meaning the electrons are in a high-energy state.
- 💥 When one of these bonds is broken, energy is released as the electrons move to a lower energy state.
- 🌊 Hydrolysis occurs when ATP is in the presence of water, resulting in the release of one phosphate group and forming ADP (Adenosine Diphosphate).
- 🔄 The conversion from ATP to ADP releases energy that can be used for various biological processes.
- 🌱 In processes like photosynthesis, light energy is used to reattach the phosphate group to ADP, forming ATP and storing energy.
- 🔥 The energy released from ATP hydrolysis can be used to generate heat or power other biological reactions.
- 🔗 ATP is essential for biological systems to function, enabling the storage and release of energy as needed.
- 🔧 Energy from ATP can change the shape of proteins or drive other reactions in the body.
Q & A
What is ATP and why is it referred to as the 'currency of energy'?
-ATP, or adenosine triphosphate, is a molecule that stores and transfers energy within biological systems. It's referred to as the 'currency of energy' because it provides energy for various cellular processes by releasing energy when one of its high-energy phosphate bonds is broken.
What are the main components of ATP?
-ATP consists of an adenosine molecule, which is made of adenine and ribose, and three phosphoryl groups. These components are critical for its function as an energy carrier.
How does ATP store energy?
-ATP stores energy in the bonds between its phosphate groups, particularly the bonds between the second and third phosphate. These are high-energy bonds, meaning that when they are broken, energy is released.
What happens during the hydrolysis of ATP?
-During ATP hydrolysis, one of the phosphate groups is removed, turning ATP into ADP (adenosine diphosphate). This process releases energy because the electrons move from a high-energy state to a more stable, lower-energy state.
What is the difference between ATP and ADP?
-ATP (adenosine triphosphate) contains three phosphate groups, while ADP (adenosine diphosphate) contains two phosphate groups. ATP stores more energy, which is released when it is converted to ADP.
What role do the high-energy bonds in ATP play?
-The high-energy bonds in ATP store energy that can be released when the bond is broken, allowing the energy to be used for various cellular processes such as muscle contraction, protein synthesis, and cell signaling.
What analogy is used to explain the concept of high-energy states in the script?
-The analogy of being in a plane at a high altitude is used. Just as a person jumping out of a plane releases energy as they fall to a lower altitude, the electrons in ATP bonds release energy as they move to a lower, more stable state.
How is ATP involved in processes like photosynthesis?
-In processes like photosynthesis, energy from light is used to add a phosphate group to ADP, converting it into ATP. This stores energy that can later be used by the cell.
What happens to the energy released from ATP hydrolysis?
-The energy released from ATP hydrolysis can be used in multiple ways, such as generating heat or driving other biochemical reactions. It can also change the shape of proteins or power cellular movements.
Why is ATP regeneration important for cellular functions?
-ATP regeneration is essential because cells constantly need energy for various functions. Processes like photosynthesis or cellular respiration replenish ATP by adding a phosphate group back to ADP, ensuring a continuous supply of energy for the cell.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

ATP: adenosín trifosfato | Energía y enzimas | Biología | Khan Academy en Español

IB Sports, exercise and health science 2024: Energy systems

Introduction of Bioenergetics Part 1

ATP Cycle
What ATP is and How it Works - BioVid Episode 3

ATP - ADENOSINA TRIFOSFATO - ESTRUTURA E FUNÇÃO | Biologia com Samuel Cunha
5.0 / 5 (0 votes)
