Mole to particle Calculations
Summary
TLDRThis chemistry video script focuses on calculations involving moles, the SI unit for quantity of particles. It explains that one mole equals 6.02 x 10^23 representative particles, which can be atoms for elements, molecules for compounds, or formula units for ionic compounds. The script demonstrates how to calculate the number of particles in a given number of moles and vice versa, using the Avogadro's number. It also highlights the importance of using the 'ee' function on calculators for handling large exponents and maintaining significant figures in calculations.
Takeaways
- 🔬 A mole is a unit in chemistry that represents 6.02 x 10^23 representative particles.
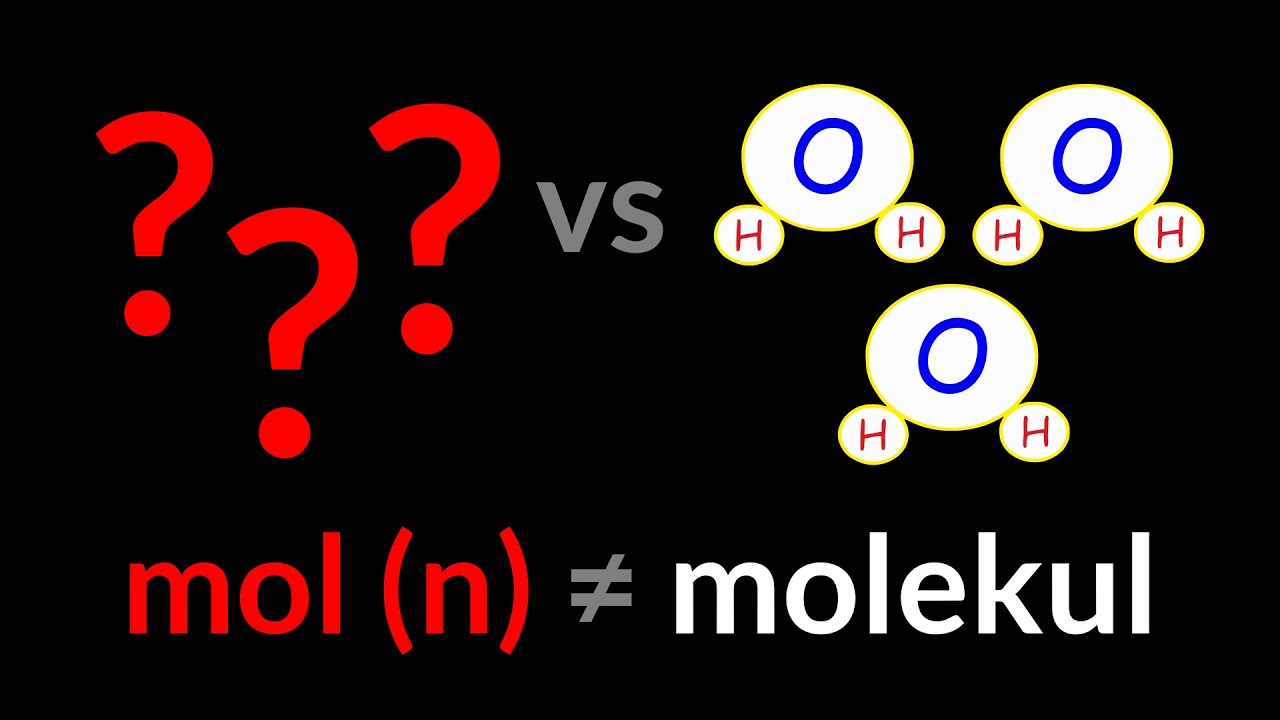
- 🌐 The term for the smallest particle depends on the substance: atoms for elements, molecules for compounds, and formula units for ionic compounds.
- 🧭 The mole concept is used to calculate the quantity of atoms, molecules, or formula units in a given amount of substance.
- 📐 The script emphasizes the importance of using the 'ee' button on calculators for handling large numbers in calculations.
- 🔢 When calculating the number of representative particles in a substance, set up a proportion using the known value of Avogadro's number (6.02 x 10^23).
- ↔️ The script demonstrates both 'moles to particles' and 'particles to moles' calculations, showing they are essentially the same mathematical process.
- 🧮 For 'moles to particles', multiply the number of moles by Avogadro's number; for 'particles to moles', divide the number of particles by Avogadro's number.
- 💡 The 'ee' function on calculators simplifies calculations by directly inputting the power of 10, avoiding manual entry of '10^'.
- ⚖️ Units are crucial in these calculations, as they determine whether you are dealing with atoms, molecules, or formula units.
- 📚 The script is educational, aiming to teach chemistry students how to perform calculations involving moles and representative particles.
Q & A
What is the significance of the number 6.02 x 10^23 in chemistry?
-The number 6.02 x 10^23 represents Avogadro's number, which is the number of representative particles in one mole of a substance.
What are representative particles?
-Representative particles are the smallest units of a substance, such as atoms for elements, molecules for molecular compounds, and formula units for ionic compounds.
How are atoms, molecules, and formula units abbreviated in chemistry?
-Atoms are abbreviated as 'atom', molecules as 'molec', and formula units as 'fu'.
What is the difference between molecular compounds and ionic compounds?
-Molecular compounds consist of molecules, typically non-metals, while ionic compounds consist of formula units formed by the electrostatic attraction between cations and anions.
How do you calculate the number of atoms in a given number of moles of an element?
-You set up a proportion with the number of moles given and Avogadro's number (6.02 x 10^23 atoms/mole), then cross multiply to find the number of atoms.
What is the purpose of the 'ee' button on a calculator in the context of chemistry calculations?
-The 'ee' button is used to input large exponents more efficiently, such as Avogadro's number, by allowing you to input the base number followed by 'ee' and then the exponent.
How do you calculate the number of formula units in a given number of moles of an ionic compound?
-The calculation is similar to that for atoms, using Avogadro's number (6.02 x 10^23 formula units/mole) and setting up a proportion.
What is the significance of significant figures in chemistry calculations?
-Significant figures indicate the precision of a measurement or calculation. In the context of this script, the number of significant figures in the final answer should match the number in the given data.
How do you calculate the number of moles from a given number of atoms?
-You divide the given number of atoms by Avogadro's number (6.02 x 10^23 atoms/mole), ensuring to use the correct function on the calculator to handle the large exponent.
Why is it important to use the correct function on the calculator when doing moles to particles or particles to moles calculations?
-Using the correct function ensures accurate handling of large exponents and prevents calculation errors, which are common when dealing with Avogadro's number.
What is the abbreviation for 'mole' in most chemistry textbooks?
-The abbreviation for 'mole' is 'mol'.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频
Stoikiometri 1 (mol adalah jumlah)

Avogadro's Number, The Mole, Grams, Atoms, Molar Mass Calculations - Introduction

Konsep Mol | Kimia SMA | Tetty Afianti

KONSEP MOL PART 1 (JUMLAH PARTIKEL)

KONSEP MOL & JUMLAH PARTIKEL : KIMIA SMA KELAS 10

Konsep Mol - perhitungan kimia / stoikiometri- kimia SMA kelas 10 semester 2
5.0 / 5 (0 votes)
