Boyle's Law | Chemistry
Summary
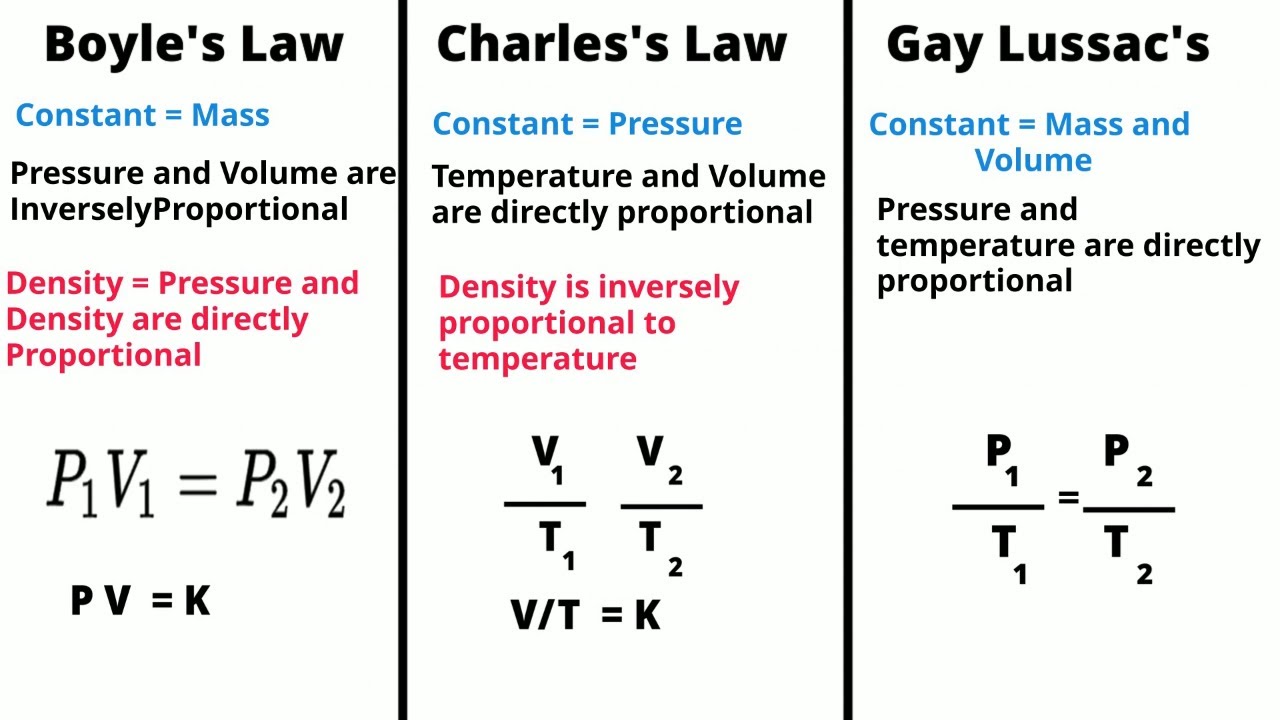
TLDRThis script covers Boyle's Law, which explores the inverse relationship between the pressure and volume of a gas, holding temperature constant. It explains key concepts such as gas molecules moving randomly, collisions with container walls, and the behavior of gases under changing conditions. The script provides examples from everyday life, like inhalation and exhalation, to demonstrate how Boyle's Law applies to real-world phenomena. Additionally, it highlights important historical references, including the contributions of British scientist Robert Boyle in establishing this fundamental principle of gas behavior.
Takeaways
- 🌡️ The experiment discusses the relationship between gas pressure and volume, highlighting the inverse proportionality when temperature is constant.
- 📊 The script mentions Boyle's Law, which is graphically represented and is fundamental to understanding gas behavior.
- 🔍 Factors affecting the behavior of gases, such as temperature and volume, are explored, emphasizing their impact on gas molecules.
- 📚 A teaching stand is mentioned for PTI Day, suggesting an educational context for the experiment.
- 💬 The script discusses the importance of understanding gas laws for teachers and students, indicating its relevance in educational settings.
- 🏫 The experiment involves a container with gas and a piston, demonstrating how changes in volume affect pressure.
- 🔬 It mentions the molecular behavior of gases, like moving randomly inside a container, and how this relates to Boyle's Law.
- 📉 As the volume of gas increases, the pressure decreases, which is a key point demonstrated in the experiment.
- 🏗️ The construction of a temple is used as an analogy to explain gas and pressure behavior, making the concept more relatable.
- 📈 The script also touches on the importance of understanding gas behavior in various applications, such as in engines and respiration.
Q & A
What is the main subject of the script?
-The script appears to be a transcript discussing various scientific concepts, possibly related to physics and chemistry, with a focus on experiments involving gases, pressure, volume, and temperature.
What does the term 'Boyl's Law' refer to in the context of the script?
-Boyle's Law is likely mentioned in reference to the inversely proportional relationship between the pressure and volume of a gas, assuming the temperature and the amount of gas remain constant.
What is the significance of the 'Experimental Proof of Flower' mentioned in the script?
-The 'Experimental Proof of Flower' seems to be a term used to describe a demonstration or experiment, possibly related to the properties of gases, but the exact significance is unclear without further context.
What does the script suggest about the behavior of gases in a closed container?
-The script suggests that the molecules of a gas in a closed container move randomly and collide with the walls of the container, which can affect the pressure.
What is the role of temperature in the behavior of gases as discussed in the script?
-The script implies that temperature affects the behavior of gases, possibly in relation to how it influences the speed of molecular motion and consequently the pressure and volume of the gas.
What is the concept of 'molar volume' mentioned in the script?
-The 'molar volume' refers to the volume occupied by one mole of a substance, which is a standard measurement in chemistry, especially when discussing gases.
What is the 'Pressure Forces Challenge' mentioned in the script?
-The 'Pressure Forces Challenge' seems to be a part of an experiment or a concept discussed in the script, likely involving the demonstration of how pressure is exerted by gases.
What is the significance of the '1662 British Scientists Robert Boyle' reference in the script?
-Robert Boyle is mentioned as a historical figure in science, likely in relation to his contributions to the understanding of gases and his formulation of Boyle's Law.
What does the script indicate about the relationship between pressure and volume of gases?
-The script suggests that there is an inversely proportional relationship between the pressure and volume of a gas, which is a fundamental concept in Boyle's Law.
What is the 'Inversely Proportional to the Volume' concept discussed in the script?
-The concept indicates that if the pressure on a gas is increased, its volume decreases, and vice versa, provided the temperature and the amount of gas remain constant.
What is the 'Gold Load Doctor' mentioned in the script?
-The term 'Gold Load Doctor' is unclear from the context provided, but it might refer to a specific point or concept in a scientific experiment or theory related to the script's discussion.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)