Understanding Second Law of Thermodynamics !
Summary
TLDRThis video script delves into the second law of thermodynamics, a fundamental yet complex principle often misunderstood. It aims to demystify the law by illustrating its application in determining the spontaneity of processes, such as gas mixing and chemical reactions, without relying heavily on mathematics. The script introduces the Clausius Inequality and entropy, crucial for understanding process directionality, and promises a practical experiment to solidify these concepts.
Takeaways
- 🔧 The second law of thermodynamics is a fundamental law of nature and is considered one of mankind's most valuable discoveries.
- 🤔 It can be confusing due to its complex terms and various ways it can be stated.
- 🔍 The law's main application is to determine whether a process is spontaneous or not.
- 🌬️ Examples of spontaneous processes include gases mixing, air leaking from a balloon, and heat loss from hot tea.
- 🙅♂️ The reverse of these processes does not happen spontaneously without external aid.
- 🔄 The first law of thermodynamics allows for the possibility of reverse processes due to energy conservation.
- 🧐 The second law is necessary to predict the direction of processes that cannot be intuitively determined, like chemical reactions.
- 📚 The video will review the second law and apply it to a chemical reaction example.
- 📐 There are two standard definitions of the second law, but they are not directly useful for engineers.
- 🌡️ The Clausius Inequality is a useful form of the second law for engineers, involving cyclic processes and temperature boundaries.
- 🔄 The inequality states that for a cyclic process, the sum of heat transferred divided by temperature is less than or equal to zero.
- 🆕 Entropy is introduced as a term to make the Clausius inequality more applicable.
Q & A
What is the Second Law of Thermodynamics?
-The Second Law of Thermodynamics is a fundamental law of nature that governs the direction of processes in nature, stating that natural processes tend to increase the total entropy of an isolated system.
Why is the Second Law of Thermodynamics considered confusing?
-It is considered confusing because it involves complex terms and can be stated in various ways, leading to difficulty in understanding its applications for most engineers and students.
What is one of the main uses of the Second Law of Thermodynamics?
-One of the main uses is to determine whether a process is spontaneous or not, meaning it can occur without any external influence.
Can the Second Law of Thermodynamics predict the direction of all processes?
-While it can predict the direction of many processes, it is not always intuitive and requires understanding the law to predict the direction of processes like chemical reactions.
What is an example of a spontaneous process mentioned in the script?
-Examples include two gases mixing together, air leaking from a balloon, a mass falling down, and hot tea losing heat.
What is an example of a non-spontaneous process mentioned in the script?
-A non-spontaneous process would be mixed gas becoming unmixed without any external aid.
How does the Second Law of Thermodynamics relate to the First Law of Thermodynamics?
-While the First Law of Thermodynamics, which is about energy conservation, states that both forward and reverse processes are possible, the Second Law introduces the concept that not all processes will occur spontaneously.
What is the Clausius Inequality and how does it relate to the Second Law?
-The Clausius Inequality is a mathematical expression that states the integral of the heat divided by temperature over a cyclic process is less than or equal to zero. It is a useful form of the Second Law for engineers, relating to the direction of heat transfer in cyclic processes.
What is entropy and how is it introduced in the context of the Second Law?
-Entropy is a thermodynamic property that measures the degree of randomness or disorder in a system. It is introduced as a term to make the Clausius Inequality more application-oriented and to understand the Second Law in terms of energy dispersal.
How does the script suggest we can better understand the Second Law of Thermodynamics?
-The script suggests that we can better understand the Second Law by learning it well and applying it to real-world examples, such as the chemical reaction mentioned.
What is the significance of the Second Law in predicting the direction of chemical reactions?
-The Second Law is significant because it helps predict whether a chemical reaction will proceed spontaneously or not, which cannot be determined by intuition alone.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

A ENTROPIA EXPLICADA

ChE 2110 Chapter 4d The Carnot Cycle

Termodinamika • Part 4: Hukum Kedua Termodinamika, Mesin Carnot & Mesin Pendingin

The Most Misunderstood Concept in Physics

THE LAWS OF THERMODYNAMICS (Zeroth law, 1st, 2nd and 3rds law) | ENGINEERING THERMODYNAMICS |
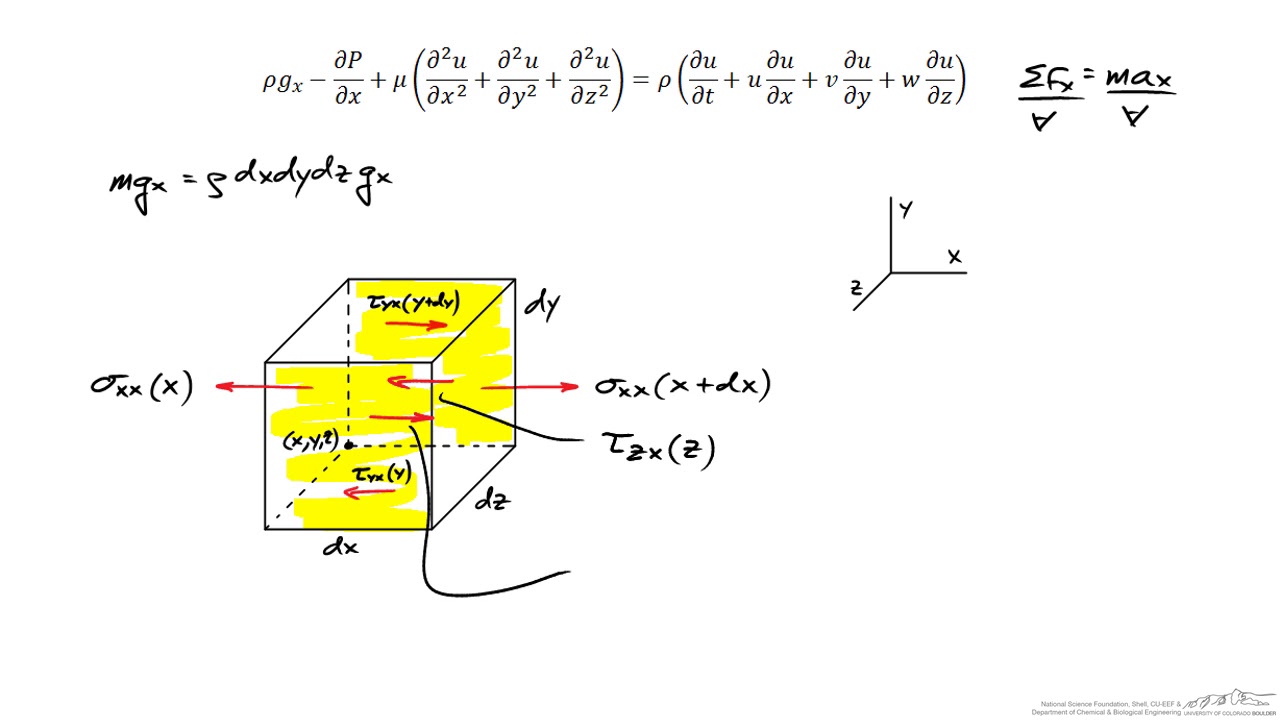
Description and Derivation of the Navier-Stokes Equations
5.0 / 5 (0 votes)
