BTECH PHYSICS | DAVISSON GERMER EXPERIMENT |APPLIED PHYSICS|DUAL NATURE of Radiation Telugu #btech
Summary
TLDRThe script discusses the Davisson-Germer experiment, a pivotal study in quantum physics that confirmed the wave-particle duality of electrons. Conducted in 1927, the experiment involved electron diffraction, leading to the 1937 Nobel Prize in Physics. The script details the experimental setup, including a filament, high-tension battery, and nickel crystal target, and explains the process of electron acceleration, diffraction, and detection. It also covers the theoretical background, including Bragg's law and its application to determine the wavelength of electrons, ultimately supporting the wave nature of matter.
Takeaways
- 🔬 The Davisson-Germer experiment of 1927 provided crucial evidence for the wave nature of electrons, supporting the de Broglie hypothesis.
- 🏆 The experiment led to the 1937 Nobel Prize in Physics for Clinton Davisson and George Paget Thomson, recognizing their work on electron diffraction.
- 🧲 The experiment involved using a high-voltage electron gun to fire electrons at a nickel target, demonstrating constructive and destructive interference patterns.
- 🎯 The diffraction patterns observed were analyzed using Bragg's law, which relates the angle of incidence to the spacing between atomic layers in the crystal.
- 📊 The experiment showed that the wavelength of electrons could be calculated using the Bragg's equation, confirming the wave-particle duality.
- 📉 The script describes a practical setup for the experiment, including a filament, high-voltage power supply, and a nickel crystal target.
- 📚 The experiment's results were graphically represented, with intensity plotted against the scattering angle, showing peaks at specific angles.
- 🔢 The script mentions the calculation of the first order maximum, which is critical for determining the wavelength of electrons and the spacing between atomic layers.
- 🏛️ The experiment's success was a significant milestone in quantum mechanics, providing a practical demonstration of the wave nature of matter.
- 📝 The script concludes with a call to action for viewers to subscribe to the channel for more educational content.
Q & A
What is the main topic of the script?
-The main topic of the script is the Davisson-Germer experiment, which demonstrated the wave nature of electrons and contributed to the development of quantum mechanics.
Who were the scientists involved in the experiment discussed in the script?
-The scientists involved in the experiment were Clinton Davisson and Lester Germer.
What was the year when the Davisson-Germer experiment was conducted?
-The Davisson-Germer experiment was conducted in 1927.
What was the purpose of the Davisson-Germer experiment?
-The purpose of the experiment was to demonstrate the wave nature of electrons by observing electron diffraction patterns, which would support the de Broglie hypothesis.
What did the de Broglie hypothesis propose?
-The de Broglie hypothesis proposed that all matter, including electrons, has wave-like properties.
What was the significance of the results from the Davisson-Germer experiment?
-The results confirmed the wave nature of electrons, providing experimental evidence for quantum mechanics and the wave-particle duality of electrons.
What Nobel Prize did the scientists receive for their work?
-Clinton Davisson and George P. Thomson (who independently conducted similar experiments) were awarded the Nobel Prize in Physics in 1937.
What was the experimental setup described in the script?
-The experimental setup involved a filament heated by a tension battery to release electrons, which were then accelerated and directed towards a nickel crystal target. The reflected electrons were observed on a collector, and the intensity of the electron beam was measured at different angles.
What is meant by 'Bragg's Law' mentioned in the script?
-Bragg's Law, nλ = 2d sinθ, is a principle used to determine the angles for constructive interference of waves, such as X-rays or electrons, reflected from a crystal lattice.
How was the wavelength of electrons calculated in the experiment?
-The wavelength of electrons was calculated using Bragg's Law, where the angle of maximum electron intensity was measured, and the known interplanar spacing of the nickel crystal was used to find the electron wavelength.
What was the role of the high tension battery in the experiment?
-The high tension battery was used to accelerate the electrons, which allowed for the observation of electron diffraction patterns when they interacted with the nickel crystal.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

Davisson Germer Experiment

Davisson-Germer Experiment & Wave-Particle Duality

Física Quântica (Dr. Quantum) Fenda Dupla - Dublado PT

Particles and waves: The central mystery of quantum mechanics - Chad Orzel

Menjawab Misteri Besar Fisika: Apakah Cahaya Gelombang atau Partikel?
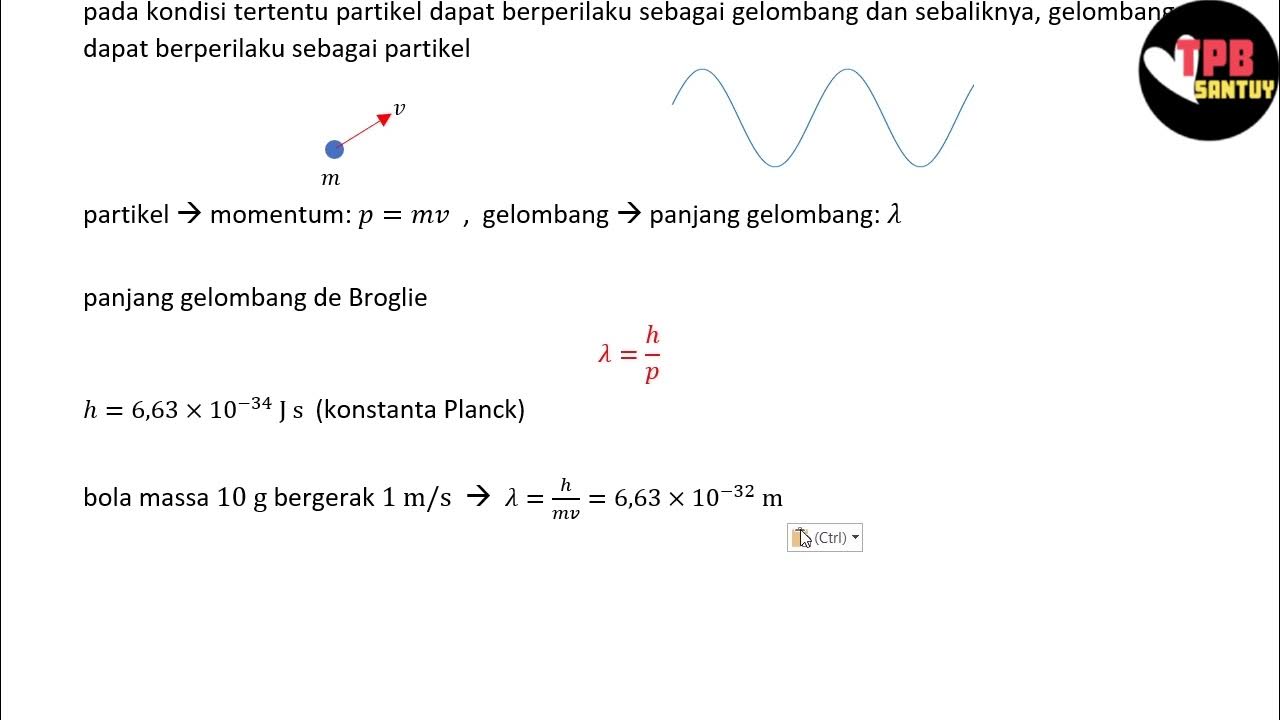
Dualisme Gelombang-Partikel | Fenomena Kuantum | Part 1 | Fisika Dasar
5.0 / 5 (0 votes)
