Chemical Bonding Explained | Ionic, Covalent and Metallic | GCSE Chemistry
Summary
TLDRThis video script delves into the three primary types of chemical bonding: ionic, covalent, and metallic. Ionic bonds form between metals and non-metals, as seen in sodium chloride, where electrons are transferred to achieve full outer shells. Covalent bonds involve electron sharing, exemplified by water, where atoms share pairs to complete their valence shells. Metallic bonding occurs in metals like iron, where a 'sea' of delocalized electrons surrounds positively charged nuclei, creating strong bonds. The video highlights the unique properties of each bond type, such as high melting points in ionic and metallic bonds, and lower boiling points in simple covalent compounds.
Takeaways
- 🔬 Ionic bonding occurs between metal and non-metal atoms, with metals losing electrons and non-metals gaining them to achieve a full outer shell.
- 💫 Metal atoms typically have 1-3 electrons in their outer shell, while non-metals have 4-8, with examples being sodium and chlorine respectively.
- 🌐 The ionic bond is formed due to the electrostatic attraction between the oppositely charged ions, resulting in a stable lattice structure.
- 🔥 Ionic compounds have high melting points because breaking the ionic bonds in the lattice requires a significant amount of energy.
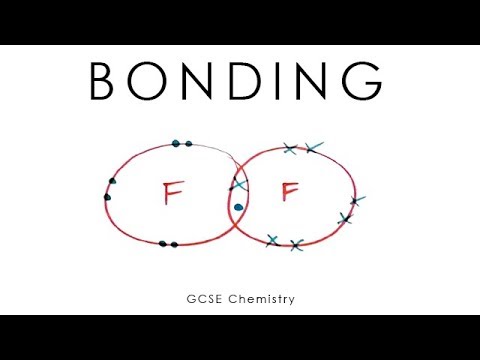
- 💧 Covalent bonding involves the sharing of electrons between non-metal atoms, as seen in water where oxygen shares with hydrogen to fill their outer shells.
- 🌌 Covalent bonds are strong, but the intermolecular forces in simple covalent compounds like water are weak, leading to low boiling points.
- 💎 In giant covalent compounds, such as diamond, the strong covalent bonds result in very high melting points due to the extensive network of shared electrons.
- 🌟 Metallic bonding is unique to metals, where a 'sea' of delocalized electrons is formed, allowing electrons to move freely among positively charged metal ions.
- 🔩 The metallic bond is strong due to the attraction between the positively charged metal ions and the delocalized electrons, giving metals high melting points.
- 📚 The video script provides a clear explanation of the three types of chemical bonds, including examples and their properties.
- 👍 The script encourages viewers to watch a follow-up video for a closer look at covalent compounds and to engage with the content by liking and subscribing.
Q & A
What are the three types of chemical bonding explained in the video?
-The three types of chemical bonding explained are ionic, covalent, and metallic bonding.
Which atoms typically engage in ionic bonding and why?
-Metal and non-metal atoms engage in ionic bonding because metals usually have 1-3 electrons in their outer shell and tend to lose them, while non-metals have 4-8 and tend to gain electrons to complete their outer shell.
How does the ionic bond form between a metal and a non-metal atom?
-The ionic bond forms when a metal atom donates its outer electron to a non-metal atom, resulting in a positively charged metal ion and a negatively charged non-metal ion that attract each other.
What is a giant lattice structure and how is it related to ionic compounds?
-A giant lattice structure is a three-dimensional arrangement of ions in an ionic compound where many ions are attracted to each other, creating a strong and stable structure.
Why do ionic compounds have high melting points?
-Ionic compounds have high melting points because a lot of energy is required to break the strong ionic bonds acting in all directions within the lattice structure.
What is covalent bonding and how does it differ from ionic bonding?
-Covalent bonding is the sharing of electron pairs between atoms, typically non-metals, to achieve a stable electron configuration. It differs from ionic bonding, which involves the transfer of electrons and the formation of ions.
Which type of covalent compound is water and why does it have a low boiling point?
-Water is a simple covalent compound. It has a low boiling point because the intermolecular forces between the water molecules are relatively weak compared to the strong covalent bonds within the molecule.
What is a giant covalent compound and how does its melting point differ from that of a simple covalent compound?
-A giant covalent compound is a large network of atoms held together by covalent bonds, like diamond. It has a very high melting point due to the extensive and strong covalent bonding throughout the structure, unlike simple covalent compounds which have weaker intermolecular forces.
What is metallic bonding and how does it involve the electrons of metal atoms?
-Metallic bonding is the type of bonding between metal atoms where a 'sea' of delocalized electrons is formed, which are free to move throughout the structure, held in place by the positively charged metal nuclei.
Why do metals generally have high melting points?
-Metals have high melting points because the metallic bond, involving the delocalized electrons and positively charged nuclei, is very strong, requiring a significant amount of energy to break.
What can viewers expect from the following video mentioned in the script?
-Viewers can expect a closer look at covalent compounds with examples to further enhance their understanding of the topic.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频
5.0 / 5 (0 votes)