Worked examples: Finding the hybridization of atoms in organic molecules | Khan Academy
Summary
TLDRThis educational video script explores the concept of hybridization in organic chemistry, providing step-by-step examples to identify hybridization states and predict molecular geometries for various atoms, excluding hydrogen. It explains the process of determining hybridization through steric numbers and sigma/pie bond counts, illustrating the geometries such as trigonal planar, tetrahedral, linear, and trigonal pyramidal. The script also emphasizes the importance of symmetry in simplifying the analysis of molecules like diethyl ether.
Takeaways
- 🧬 The script discusses the concept of hybridization and its importance in understanding molecular geometry.
- 🔍 To identify hybridization, one can look at the type of bonds (single, double, triple) associated with an atom.
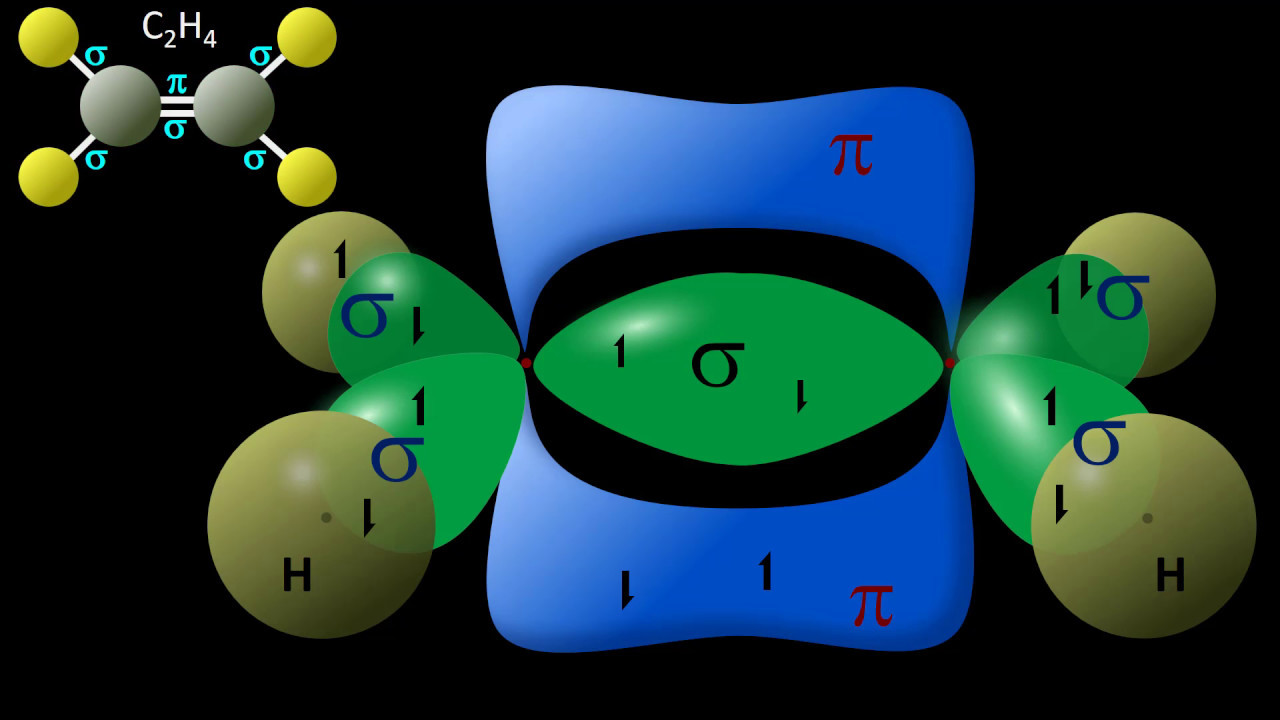
- 📊 SP2 hybridization is associated with a trigonal planar geometry and bond angles of approximately 120 degrees.
- 📐 SP3 hybridization results in a tetrahedral geometry with ideal bond angles of 109.5 degrees.
- 🔬 SP hybridization leads to a linear geometry with a bond angle of 180 degrees.
- 📝 The total number of sigma and pi bonds in a molecule can be counted to further understand its structure.
- 🌈 Sigma bonds are represented in blue, while pi bonds are in red in the script's illustrations.
- 🔢 The steric number, calculated as the sum of sigma bonds and lone pairs, helps determine the hybridization state of an atom.
- 🌀 Hydrogen atoms are excluded from hybridization analysis as they are only bonded to one other atom and do not have a significant geometry.
- 🧩 In the example of diethyl ether, all carbon atoms are SP3 hybridized with a tetrahedral geometry, while the oxygen is SP3 hybridized but has a bent geometry due to lone pairs.
- 🌟 The script emphasizes the importance of symmetry in simplifying the analysis of molecular geometry, as seen in the diethyl ether example.
Q & A
What is the primary method discussed in the script for identifying the hybridization state of carbon atoms?
-The primary method discussed is to look at the type of bonds a carbon atom has. For example, if a carbon has a double bond, it is SP2 hybridized; if it has a triple bond, it is SP hybridized; and if it only has single bonds, it is SP3 hybridized.
What is the geometrical shape associated with SP2 hybridization?
-The geometrical shape associated with SP2 hybridization is trigonal planar, with bond angles approximately 120 degrees.
How many sigma and pi bonds are there in a double bond?
-A double bond consists of one sigma bond and one pi bond.
What is the hybridization state of a carbon atom with only single bonds around it?
-A carbon atom with only single bonds around it is SP3 hybridized.
What is the ideal bond angle in a tetrahedral geometry?
-The ideal bond angle in a tetrahedral geometry is 109.5 degrees.
What is the hybridization state of a carbon atom with a triple bond?
-A carbon atom with a triple bond is SP hybridized.
What is the geometrical shape associated with SP hybridization?
-The geometrical shape associated with SP hybridization is linear, with a bond angle of 180 degrees.
How many sigma bonds are there in the molecule discussed in the script?
-There are a total of 10 sigma bonds in the molecule discussed in the script.
How many pi bonds are there in the molecule discussed in the script?
-There are three pi bonds in the molecule discussed in the script.
What is the steric number and how is it used to determine hybridization states?
-The steric number is the sum of the number of sigma bonds and lone pairs of electrons around an atom. It is used to determine the hybridization state by indicating the number of hybrid orbitals needed to accommodate the electron groups.
Why are hydrogen atoms excluded from the hybridization and geometry analysis in the script?
-Hydrogen atoms are excluded because they are only bonded to one other atom and do not have a geometry that can be analyzed in the same way as other atoms with multiple bonds or lone pairs.
What is the hybridization state of the oxygen atom in diethyl ether, and what is its geometry?
-The oxygen atom in diethyl ether is SP3 hybridized, and its geometry is bent or angular, not tetrahedral, due to the presence of two lone pairs of electrons.
What is the difference between the electron group geometry and the molecular geometry around an SP3 hybridized atom?
-The electron group geometry refers to the arrangement of all electron groups (including lone pairs) around an atom, which for SP3 hybridization would be tetrahedral. However, the molecular geometry only considers the positions of the atoms bonded to the central atom, which can be different from the electron group geometry due to the presence of lone pairs.
What is the hybridization state and geometry of the nitrogen atom in the last example of the script?
-The nitrogen atom in the last example is SP3 hybridized, but its geometry is trigonal pyramidal due to the presence of three sigma bonds and one lone pair of electrons.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Hibridisasi SP, SP2 dan SP3

Organic Chemistry 1, Chapter 1-2: Structure & Bonding

Valence Bond Theory & Hybrid Atomic Orbitals

More Organic Nomenclature: Heteroatom Functional Groups: Crash Course Organic Chemistry #3

Hybridation des orbitales atomiques (3) - Tableau récapitulatif
Hybrid Orbitals explained - Valence Bond Theory | Orbital Hybridization sp3 sp2 sp
5.0 / 5 (0 votes)