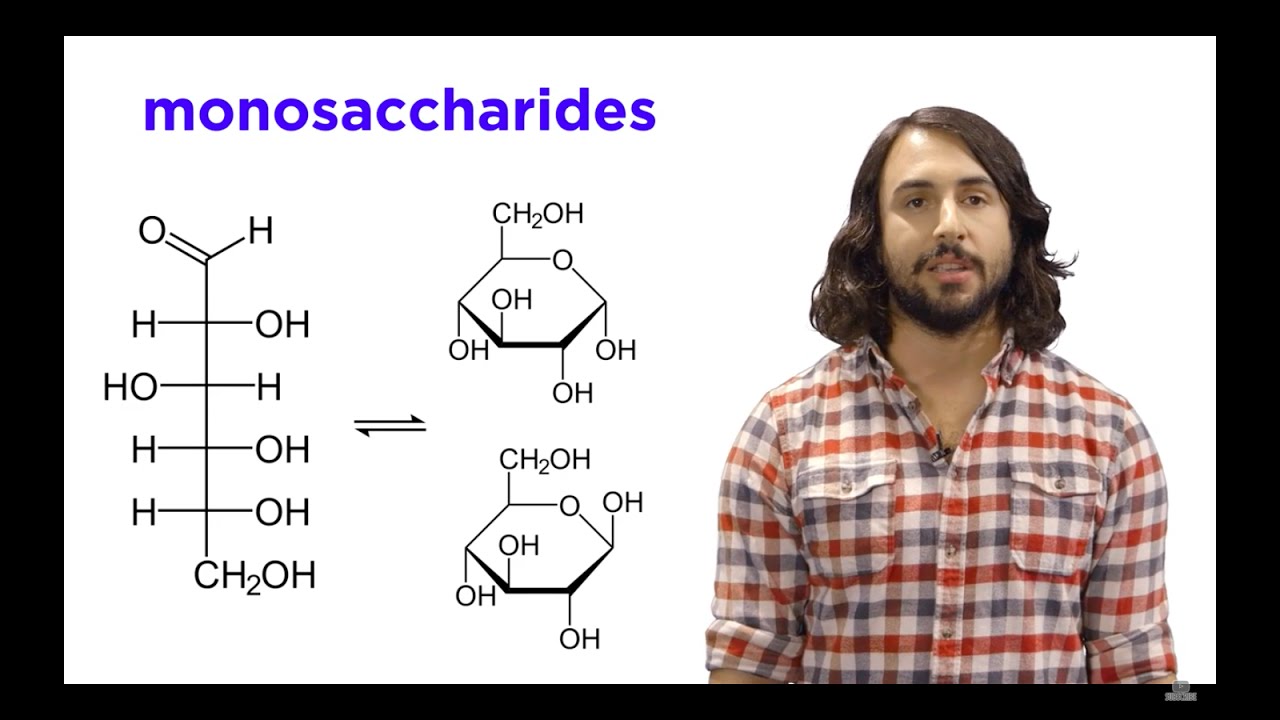
Carbohydrates! D vs L Stereoisomers
Summary
TLDRThis educational video script delves into the identification of D and L stereoisomers in carbohydrate chemistry. It emphasizes the importance of correctly numbering carbons, ensuring the carbonyl carbon receives the lowest number, and identifying chiral carbons. The script guides viewers on how to determine whether a sugar is D or L by examining the orientation of the hydroxyl group on the highest numbered chiral carbon. It also suggests flipping the molecule if necessary to align with standard representation for accurate determination. The script concludes with a practice exercise for viewers to apply their newly acquired knowledge.
Takeaways
- 📝 To identify D or L stereoisomers in carbohydrates, one must be able to number the carbons correctly and identify chiral carbons.
- 🔢 The carbonyl carbon should always be assigned the lowest possible number, which is typically achieved by numbering from top to bottom or bottom to top.
- 🤔 Proper numbering is crucial as incorrect numbering can lead to identifying the wrong chiral carbons.
- 🔍 Chiral carbons are essential to determine the stereochemistry of a carbohydrate, and there are two in the example provided in the script.
- 📚 If the orientation of the sugar is not standard, it may be necessary to rotate or flip the molecule to align with the typical representation for analysis.
- 👀 The highest numbered chiral carbon is the key to determining if a sugar is D or L form.
- ➡️ For the D form, the hydroxyl group (OH) on the highest numbered chiral carbon should be on the right side when viewed correctly.
- ⬅️ Conversely, for the L form, the hydroxyl group (OH) should be on the left side of the highest numbered chiral carbon.
- 🔁 Drawing the mirror image of a sugar molecule will result in its enantiomer, which is the opposite stereoisomer (D or L).
- 📐 The script provides a practical approach to practicing the identification of stereoisomers by encouraging viewers to pause and attempt the task themselves.
- 📘 The script also suggests that sugars are typically drawn with the number one carbon at the top and the highest numbered carbon at the bottom for ease of stereoisomer identification.
Q & A
What is the primary focus of the video?
-The video focuses on understanding D and L stereoisomers in the context of carbohydrate chemistry.
What are the two essential steps to identify whether a carbohydrate is a D or L stereoisomer?
-The two essential steps are: 1) Numbering the carbons appropriately, ensuring the carbonyl carbon gets the lowest number possible. 2) Identifying the chiral carbons.
Why is it crucial to number the carbons correctly in determining the stereoisomer of a carbohydrate?
-Correct numbering is crucial because it helps in identifying the highest numbered chiral carbon, which is key in determining whether the carbohydrate is a D or L stereoisomer.
What should be the lowest numbered carbon in a carbohydrate molecule when determining stereoisomers?
-The carbonyl carbon should be the lowest numbered carbon in a carbohydrate molecule.
How can you identify chiral carbons in a carbohydrate molecule?
-Chiral carbons are identified by looking for carbon atoms that have four different groups attached to them. The video suggests a video link for further information on identifying chiral carbons.
What is the significance of the highest numbered chiral carbon in determining the D or L form of a carbohydrate?
-The highest numbered chiral carbon is significant because the position of the hydroxyl group (OH) on this carbon determines whether the carbohydrate is a D or L stereoisomer.
What is the rule for the orientation of the carbohydrate molecule when determining its stereoisomer form?
-The rule is that the number one carbon should be at the top and the plus-numbered carbons should be at the bottom when determining the stereoisomer form.
How does the position of the hydroxyl group on the highest numbered chiral carbon help in determining the stereoisomer form of a carbohydrate?
-If the hydroxyl group is on the right, it is the D form, and if it is on the left, it is the L form.
What is the practical approach to practice identifying D and L stereoisomers in carbohydrates?
-The practical approach involves numbering the carbons correctly, identifying the chiral carbons, and then examining the highest numbered chiral carbon to determine the position of the hydroxyl group.
What is the relationship between the orientation of the carbohydrate molecule and the ease of determining its stereoisomer form?
-The orientation of the molecule, particularly with the number one carbon at the top and the plus-numbered carbons at the bottom, makes it easier to apply the rules for determining the D or L form.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)