Bomb Calorimeter
Summary
TLDRThis video script explains the process of determining the calorific value of a fuel sample using a bomb calorimeter. The fuel sample is placed inside a steel bomb with electrodes and a fuse wire. Oxygen is added, and the bomb is submerged in a calorimeter containing water. The fuel is ignited by an electric current, transferring heat to the water. Temperature changes are recorded before and after combustion. Using these data, the calorific value of the fuel is calculated, considering factors like water weight, temperature changes, and the calorimeter’s water equivalent.
Takeaways
- 😀 A sample fuel of weight 'x' grams is used in a bomb calorimeter experiment to determine its calorific value.
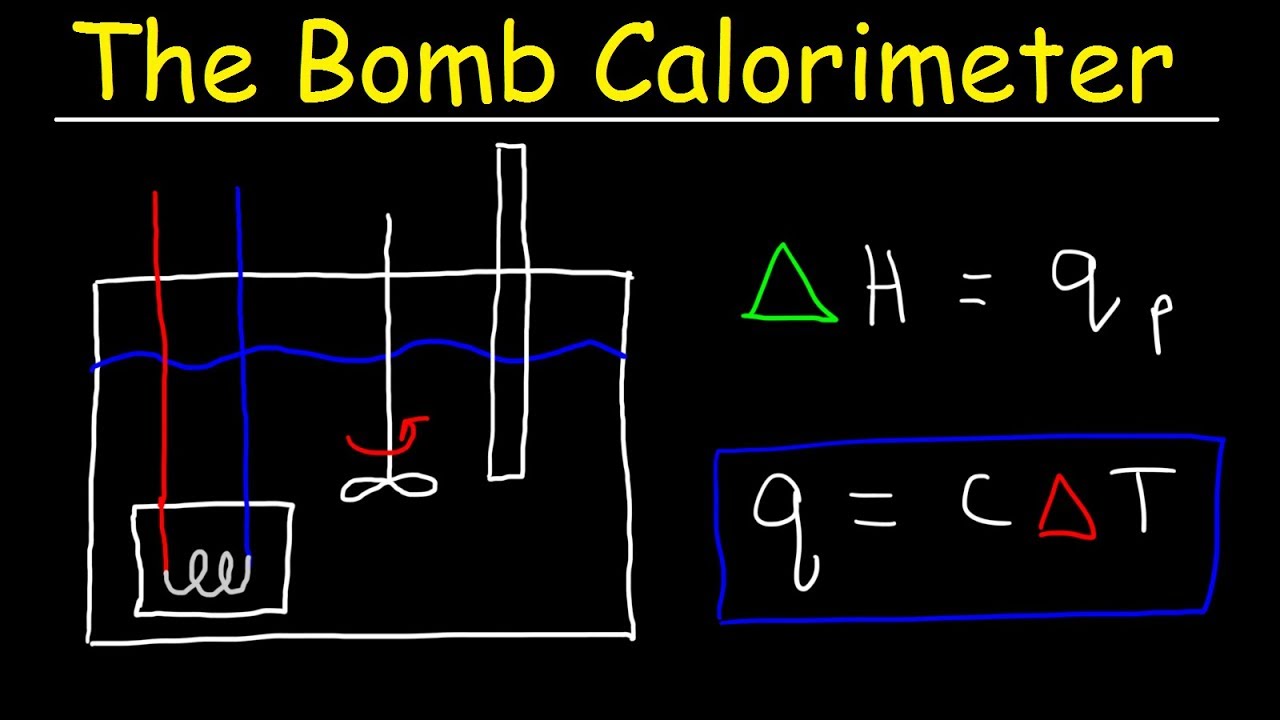
- 😀 The fuel sample is placed inside a crucible, which is then put inside a steel bomb.
- 😀 The steel bomb is sealed with a lid that includes two electrodes for conducting electricity.
- 😀 Magnesium or platinum fuse wire is tightly stretched between the electrodes to burn the fuel sample.
- 😀 The bomb is filled with a large amount of oxygen (O2) at a pressure of 25 to 30 atmospheric pressures.
- 😀 The calorimeter, which contains no water initially, is used to measure the heat transferred during the reaction.
- 😀 The initial temperature of the water in the calorimeter is recorded, typically around 21°C.
- 😀 The apparatus is covered with a lid that includes a Beckmann's thermometer and battery wires for electricity.
- 😀 A 6V battery is used to pass an electric current through the electrodes, igniting the fuel sample.
- 😀 The heat produced by burning the fuel is transferred to the surrounding water, which is stirred by an electric stirrer.
- 😀 The temperature of the water is recorded again after the reaction, yielding a final temperature (T2).
- 😀 The calorific value of the fuel is calculated using a specific formula, considering the weight of the fuel, water, and temperature change.
Q & A
What is the purpose of using a bomb calorimeter in this experiment?
-The purpose of using a bomb calorimeter in this experiment is to determine the calorific value (energy content) of a sample fuel by measuring the heat released during its combustion.
What is the initial step in preparing the bomb calorimeter?
-The initial step involves placing a known weight of the fuel sample in a crucible, which is then placed inside a steel bomb.
How is the bomb calorimeter set up for combustion?
-The bomb is sealed with a lid, and two electrodes are attached to it. Magnesium or platinum fuse wire is stretched across the electrodes. The bomb is then filled with oxygen at 25-30 atmospheric pressure.
Why is oxygen filled into the bomb calorimeter at high pressure?
-Oxygen is filled at high pressure to ensure complete combustion of the fuel sample inside the bomb, providing accurate results for the calorific value.
What role does the calorimeter play in this experiment?
-The calorimeter holds the bomb containing the fuel sample, and it is filled with water to absorb the heat released during combustion. The temperature change in the water is used to calculate the calorific value.
How is the temperature measured during the experiment?
-The temperature of the water is measured using a Beckmann thermometer, and the rise in temperature is noted before and after the combustion of the fuel sample.
What is the function of the electric stirrer in the calorimeter?
-The electric stirrer ensures that the water in the calorimeter is evenly stirred, allowing for uniform heat distribution and accurate temperature measurements.
How is the fuel sample ignited in the bomb calorimeter?
-The fuel sample is ignited by passing an electric current through the fuse wire, which burns the fuel inside the bomb.
What formula is used to calculate the calorific value, and what variables does it include?
-The calorific value is calculated using the formula: L = (W * (T2 - T1) * 4.1868) / (X), where X is the weight of the fuel sample, W is the weight of the water, T1 is the initial temperature, T2 is the final temperature, and 4.1868 is the specific heat capacity of water.
What does the term 'gross calorific value' refer to in this context?
-The gross calorific value refers to the total energy released when a fuel is completely burned, including the latent heat of water vapor formed during combustion.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
Bomb Calorimeter vs Coffee Cup Calorimeter Problem - Constant Pressure vs Constant Volume Calorimet

Uji Gross Energi

KALORIMETER : Menghitung Perubahan Entalpi dengan Kalorimetri - Kimia kelas XI

Fuels l Fuels & combustion l Characteristics of Good Fuel l Engineering Chemistry l Fuel Definition

Kadar Karbonat Metode Titrasi Asidimetri (Tutorial Prosedur Analisis + perhitungan)

Fuel Types - Fuel Systems - Airframes & Aircraft Systems #64
5.0 / 5 (0 votes)