Uji Karbohidrat - Molisch, Iodium, Barfoed, Benedict, Seliwanoff, dan Osazon
Summary
TLDRThis practical biochemistry video walks viewers through a series of carbohydrate tests. It starts with the Molisch test, a preliminary step to detect carbohydrates, followed by iodine, Barfoed, Benedict, Seliwanoff, and Osazon tests to differentiate between polysaccharides, monosaccharides, and reducing sugars. Each test is demonstrated with sample reactions and explanations of their specific purposes, from identifying starch to distinguishing aldehyde and ketone groups. The video provides a comprehensive overview of various tests used in carbohydrate analysis, making it an informative guide for practical laboratory work.
Takeaways
- 😀 Uji Molisch is a preliminary test for carbohydrates to detect the presence of carbohydrates in a sample. It forms a purple ring when positive for carbohydrates.
- 😀 Uji Iodium is used to detect polysaccharides. A positive result is indicated by a color change when iodine is added.
- 😀 Uji Barfoed distinguishes between monosaccharides and disaccharides. A red precipitate within 5 minutes indicates monosaccharides.
- 😀 Uji Benedict tests for reducing sugars, which include monosaccharides. It forms a red precipitate when positive for reducing sugars.
- 😀 Uji Selliwanoff is used to differentiate between aldose (aldehyde group) and ketose (ketone group) carbohydrates. Ketoses react faster than aldoses.
- 😀 Uji Osazon is used to identify sugars based on the crystals they form when heated, providing unique shapes for different sugars.
- 😀 The test for molisch shows that aldehydes in carbohydrates react with alpha-naphthol to produce a purple color.
- 😀 Only samples containing polysaccharides (like starch) show a positive result in the iodine test due to their unique structure.
- 😀 The Barfoed test shows that monosaccharides reduce copper ions quickly, while disaccharides do not react as fast.
- 😀 Benedict’s test reveals that only reducing sugars, such as glucose, react positively, while non-reducing sugars like sucrose do not react.
Q & A
What is the Molisch test used for in carbohydrate testing?
-The Molisch test is a preliminary test used to detect the presence of carbohydrates in a sample. A purple ring forms at the interface of two layers if carbohydrates are present.
Why does aquades (water) not react in the Molisch test?
-Aquades does not contain any carbohydrates, so no purple ring forms during the Molisch test, as the test specifically reacts with aldehyde groups present in carbohydrates.
What is the purpose of the Iodine test in carbohydrate analysis?
-The Iodine test is used to confirm the presence of polysaccharides, such as starch, which will form a specific color complex when iodine is added.
What is the significance of the blue color change in the Iodine test?
-The blue color change indicates the presence of starch, a polysaccharide composed of amylose and amylopectin. The iodine interacts with these molecules to form the color complex.
What does the Barfoed test differentiate between?
-The Barfoed test is used to differentiate between monosaccharides and disaccharides. It shows a red precipitate when monosaccharides react quickly with the solution, whereas disaccharides react more slowly.
Why does the Benedict test show positive results for reducing sugars?
-The Benedict test detects reducing sugars that contain aldehyde or ketone groups. When these sugars react with the Benedict reagent, they form a red precipitate.
What is the difference between positive and negative results in the Benedict test?
-A positive result in the Benedict test is indicated by a red precipitate, showing the presence of reducing sugars. A negative result means there is no reaction, and the sample does not contain reducing sugars like sucrose or starch.
What does the Seliwanoff test distinguish between?
-The Seliwanoff test distinguishes between aldose (aldehyde-containing sugars) and ketose (ketone-containing sugars). Ketoses react faster, producing a red color, while aldoses react more slowly.
What is the principle behind the formation of osazone crystals in the Osazon test?
-The Osazon test involves the formation of crystalline structures when carbohydrates, such as glucose or galactose, react with specific reagents. These crystals can be used for carbohydrate identification based on their shape under a microscope.
Why does maltose form osazone crystals during the Osazon test?
-Maltose, a disaccharide with a reducing sugar group, forms osazone crystals when it reacts with the Osazon reagent. These crystals can be observed under a microscope, helping identify the sugar.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
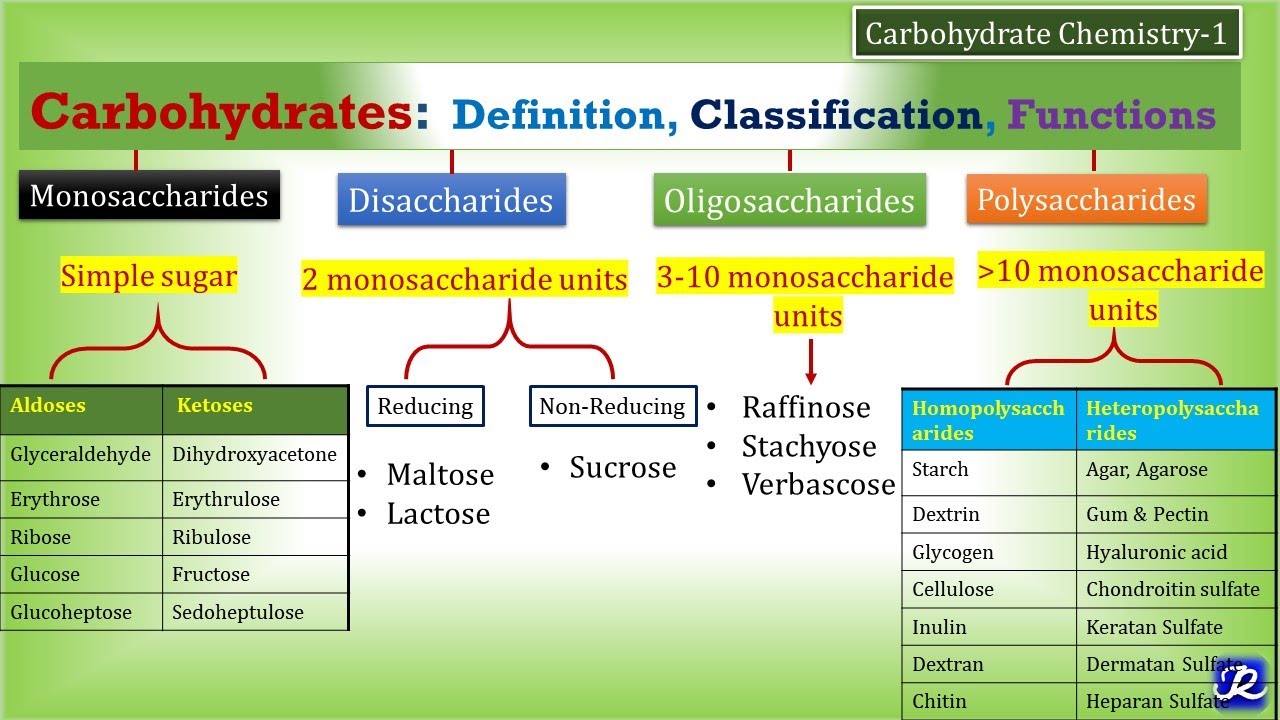
1:Carbohydrates-Definition, Classification, Functions | Carbohydrate Chemistry 1| Biochemistry

Time Series Analysis - 2 | Time Series in R | ARIMA Model Forecasting | Data Science | Simplilearn

Mikrobiologi Dasar - Uji Aktivitas Biokimia

Pola Bilangan (5) | Barisan dan Deret Geometri

Qualitative Tests for Carbohydrates (Biochemistry)

METABOLISME KARBOHIDRAT - Kuliah Online BIOKIMIA
5.0 / 5 (0 votes)