A Level Chemistry Revision (Year 13) "Directing Groups in the Reactions of Benzene" (OCR)
Summary
TLDRIn this video, we explore the influence of electron-donating and electron-withdrawing groups on the reactivity of benzene compounds. Electron-donating groups, like the hydroxy and amine groups, activate the benzene ring and direct substitution to carbons 2 and 4. In contrast, electron-withdrawing groups, like the nitro group, decrease reactivity and direct substitution to carbon 3. The video explains the importance of these directing effects when synthesizing organic molecules and provides insights into how the positioning of substituents affects chemical reactions in benzene.
Takeaways
- 😀 Benzene is relatively resistant to bromination compared to alkenes, and requires a halogen carrier catalyst, like iron bromide.
- 😀 The pi electrons in benzene are delocalized, meaning they cannot induce a dipole in bromine molecules on their own.
- 😀 In phenol, the oxygen atom donates a lone pair of electrons to the benzene ring, making it more reactive and allowing it to react with bromine without a halogen carrier.
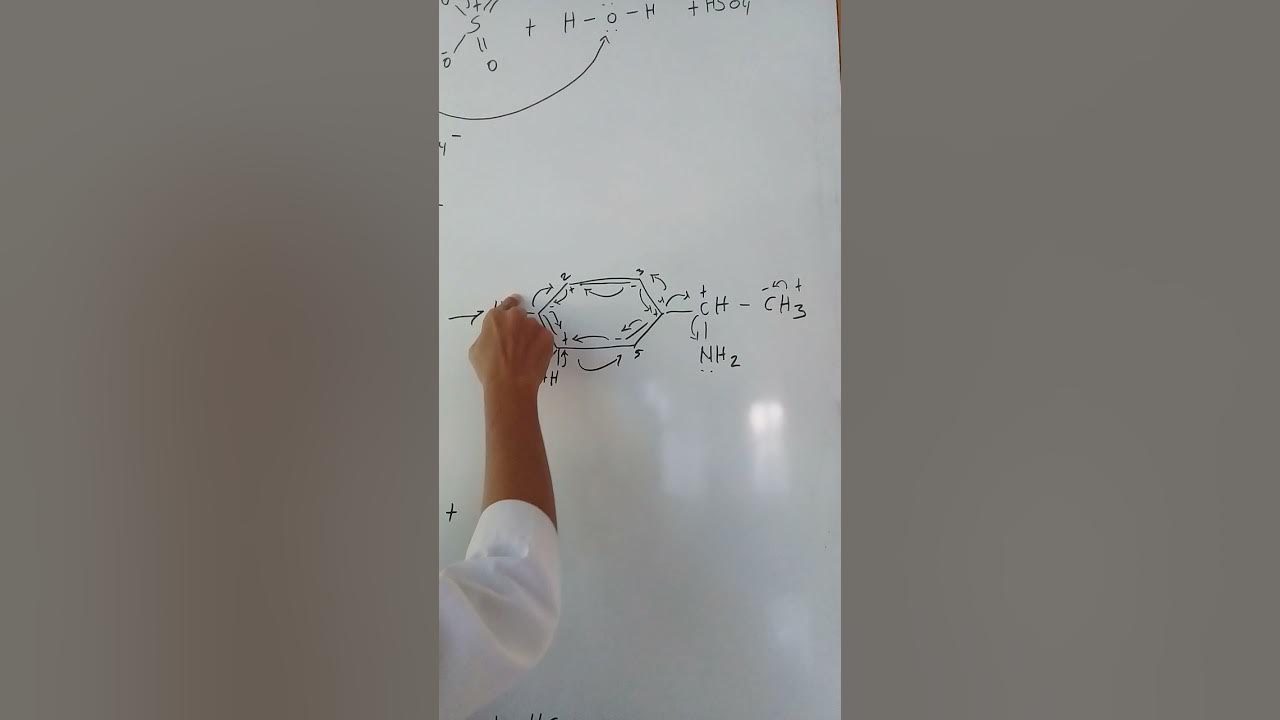
- 😀 The amine group (NH2) in phenyamine also donates electrons to the benzene ring, activating it and enabling reaction with bromine without a halogen carrier.
- 😀 Electron-donating groups like hydroxy (OH) and amine (NH2) increase the electron density on the benzene ring, making it more reactive towards electrophilic substitution reactions.
- 😀 Electron-donating groups (e.g., OH, NH2) direct substitution to carbons 2, 4, and 6 on the benzene ring.
- 😀 Symmetrical molecules like phenol and phenyamine show equivalent reactivity at carbons 2 and 6 due to symmetry in their structures.
- 😀 Nitrobenzene, which contains the electron-withdrawing NO2 group, is less reactive to bromination and requires a halogen carrier and high temperature to react.
- 😀 Electron-withdrawing groups like NO2 reduce the electron density on the benzene ring, making it less reactive and directing substitution to carbon 3.
- 😀 When synthesizing organic molecules, it’s important to consider the effects of directing groups to plan the order of reactions for desired substitutions.
- 😀 For exams, only the effects of the OH, NH2, and NO2 groups need to be known; information about other groups will be provided in the question.
Q & A
Why is benzene more resistant to bromination compared to alkenes?
-Benzene is more resistant to bromination because its pi electrons are delocalized across all six carbon atoms. This makes the electron density between adjacent carbon atoms insufficient to induce a dipole in the bromine molecule, unlike alkenes where the electron density in the double bond is enough to react with bromine under normal conditions.
What is the role of a halogen carrier catalyst in the bromination of benzene?
-A halogen carrier catalyst, such as iron bromide, is required in the bromination of benzene because it helps to generate the reactive bromine species that can induce the reaction. Without the catalyst, benzene would not react with bromine due to the lack of sufficient electron density to polarize the bromine molecule.
How does phenol differ from benzene in terms of reactivity with bromine?
-Phenol is more reactive with bromine compared to benzene because the lone pair of electrons on the oxygen atom in phenol is donated into the delocalized electron structure of the benzene ring. This increases the electron density in the ring, making it more reactive and able to induce a dipole in bromine, enabling the reaction to occur without a halogen carrier.
What is meant by an 'activating group' in the context of phenol and phenyamine?
-An activating group is a substituent that increases the electron density in the benzene ring, making the molecule more reactive. In phenol, the hydroxyl group (OH) donates electrons into the ring, and in phenyamine, the amine group (NH2) donates electrons as well. Both of these groups activate the ring and make it more likely to undergo substitution reactions with bromine.
What are the positions where substitution occurs in the presence of electron-donating groups?
-Electron-donating groups, such as the hydroxy group (OH) and the amine group (NH2), direct substitution to the 2, 4, and 6 positions on the benzene ring. In symmetrical molecules like phenol and phenyamine, carbon 6 is considered equivalent to carbon 2.
What effect do electron-withdrawing groups, like the nitro group, have on the reactivity of benzene?
-Electron-withdrawing groups, such as the nitro group (NO2), reduce the electron density in the benzene ring, making it less reactive. For instance, nitrobenzene only reacts with bromine in the presence of a halogen carrier and under high-temperature conditions.
How do electron-withdrawing groups affect the positions of substitution on the benzene ring?
-Electron-withdrawing groups direct substitution to the meta position (carbon 3) on the benzene ring. This is in contrast to electron-donating groups, which direct substitution to the ortho and para positions.
What is the significance of the O group and NH2 group in terms of the OCR specification?
-The OCR specification requires students to understand the effects of the O group (hydroxyl) and the NH2 group (amine) as electron-donating groups. These groups activate the benzene ring and direct substitution to positions 2 and 4. The NO2 group (nitro) is also covered as an electron-withdrawing group, which directs substitution to the meta position.
Why is it important to consider the order of reactions when synthesizing an organic molecule involving benzene?
-When synthesizing an organic molecule, the order of reactions is important because the positions where different groups are substituted on the benzene ring can affect the outcome of subsequent reactions. Understanding the directing effects of substituent groups ensures that the desired substitution pattern is achieved.
What are the terms 'ortho', 'meta', and 'para' positions in relation to the benzene ring?
-The terms 'ortho', 'meta', and 'para' refer to the relative positions of substituents on the benzene ring. Ortho refers to adjacent carbons (1,2), meta refers to carbons separated by one position (1,3), and para refers to carbons that are opposite each other (1,4). These positions are important in determining where substitution reactions occur on the ring.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Reaksi pada Karbonil Alfa-Beta Tidak Jenuh

Why aldehydes are more reactive than ketones towards nucleophilic addition reaction? #bepharmawise

Nucleophilic Aromatic Substitution

Reaksi Benzena | Reaksi Alkilasi Friedel-Crafts: Mekanisme Reaksi dan Batasan Alkilasi [LENGKAP]

GCSE Chemistry - Halogens and Noble Gases #12
Proses Sulfonasi Senyawa Aromatik
5.0 / 5 (0 votes)