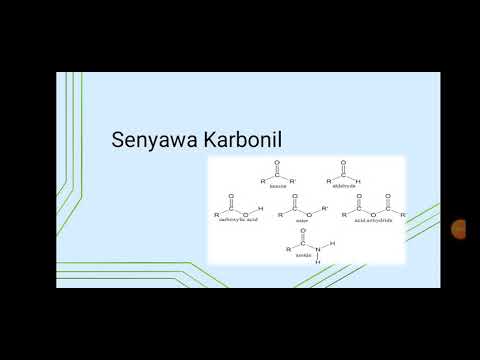
Reaksi pada Karbonil Alfa-Beta Tidak Jenuh
Summary
TLDRThis video covers a detailed exploration of organic chemistry reactions, particularly focusing on alpha-beta unsaturation in carbonyl compounds. It delves into the mechanisms of Michael addition, keto-enol tautomerization, and the reactivity of various carbonyl groups. The speaker explains the importance of electron-donating and electron-withdrawing groups, highlighting how different reagents such as NaOH, Grignard reagents, and enolate ions interact with carbonyl compounds. The tutorial also offers practical examples and problem-solving techniques for understanding and applying these concepts, providing valuable insights for students learning organic chemistry reactions.
Takeaways
- 😀 **Alpha-Beta Unsaturated Carbonyl Compounds**: These compounds feature conjugated systems with both a double bond (alkene) and a carbonyl group, which contribute to stability through resonance, making them electrophilic and reactive in various chemical reactions.
- 😀 **Michael Addition Reaction**: A key reaction where a nucleophile attacks an electrophilic carbonyl group in an alpha-beta unsaturated carbonyl compound, forming a new carbon-carbon bond. The nucleophile typically comes from compounds like NaOH or NaOCH3.
- 😀 **Resonance Stabilization**: The resonance between the carbonyl group and the alkene in alpha-beta unsaturated carbonyl compounds stabilizes the system, making it more reactive toward nucleophiles.
- 😀 **Enolate Formation**: In reactions involving strong bases, such as NaOH, enolate ions are generated by deprotonation of the alpha-hydrogen. These enolate ions are crucial intermediates in many reactions, including Michael addition.
- 😀 **Reactivity of Nucleophiles**: Nucleophiles like OH⁻ or NaOCH3 attack the electrophilic carbonyl carbon. The position of attack is influenced by the stability and structure of the alpha-beta unsaturated carbonyl compound.
- 😀 **Keto-Enol Tautomerization**: This process involves the equilibrium between the keto form and the enol form of a compound. The keto form is usually more stable due to the ability to form a strong carbonyl bond, whereas the enol form is less stable but can be involved in reactions.
- 😀 **Base-Induced Reactions**: Reactions involving bases like NaOH or NaOCH3 are essential for generating enolate intermediates. These intermediates can then react further with electrophiles in reactions like Michael addition.
- 😀 **Grignard Reagents**: The addition of Grignard reagents (e.g., RMgX) to alpha-beta unsaturated carbonyl compounds does not typically follow Michael addition but instead reacts directly with the carbonyl group to form a new carbon-carbon bond.
- 😀 **Hydrogen Alpha**: The alpha-hydrogen, the hydrogen attached to the carbon next to the carbonyl group, is more acidic compared to other hydrogens. Its deprotonation leads to the formation of enolates.
- 😀 **Reaction Pathways and Stability**: The stability of the intermediate forms, like enolates and the keto form, plays a key role in determining the reaction product. For example, enolates can be unstable and need to be stabilized or converted back to the keto form.
- 😀 **Reaction Mechanism Understanding**: Understanding the detailed mechanism of reactions involving alpha-beta unsaturated carbonyl compounds is critical. This includes recognizing the sequence of nucleophilic attacks, resonance shifts, and protonation steps that lead to the final products.
Q & A
What is the significance of alpha-beta unsaturated carbonyl compounds in organic reactions?
-Alpha-beta unsaturated carbonyl compounds are important because their conjugated structure (double bond next to a carbonyl group) makes them highly reactive. The carbonyl group is an electrophilic site, and the conjugation with the double bond allows for resonance stabilization, making these compounds susceptible to nucleophilic attacks, such as in Michael addition reactions.
What is the Michael addition reaction, and why is it important in organic chemistry?
-The Michael addition is a type of nucleophilic addition where a nucleophile attacks the electrophilic carbon in an alpha-beta unsaturated carbonyl compound. This reaction is important because it allows the formation of carbon-carbon bonds, which is a fundamental step in many organic synthesis processes, especially in the creation of complex molecules.
What role does resonance play in the stability of alpha-beta unsaturated carbonyl compounds?
-Resonance in alpha-beta unsaturated carbonyl compounds stabilizes the molecule by distributing the electron density over the structure. This makes the carbonyl carbon more electrophilic and prone to attack by nucleophiles. The resonance allows for the delocalization of the positive charge, stabilizing the intermediate during reactions.
How does the addition of a Grignard reagent differ from other nucleophilic additions to alpha-beta unsaturated carbonyl compounds?
-Grignard reagents (RMgX) are highly reactive nucleophiles with a strong negative charge on the carbon atom. When they react with alpha-beta unsaturated carbonyl compounds, they primarily attack the carbonyl carbon rather than the beta carbon. This differs from other nucleophilic additions, like Michael addition, where the nucleophile typically attacks the beta carbon, leading to different product outcomes.
Why are alpha-hydrogens considered more acidic in the context of alpha-beta unsaturated carbonyl reactions?
-Alpha-hydrogens are more acidic in alpha-beta unsaturated carbonyl compounds because the adjacent carbonyl group withdraws electron density from the alpha carbon. This makes the hydrogen on the alpha carbon more likely to dissociate as a proton, forming an enolate ion. This increased acidity makes the alpha-hydrogens more susceptible to deprotonation by strong bases.
What is the keto-enol tautomerism, and why is the keto form generally more stable?
-Keto-enol tautomerism refers to the equilibrium between the keto form (where the carbonyl group is present) and the enol form (where the hydroxyl group is attached to a double bond). The keto form is typically more stable because the carbonyl group is a more stable functional group compared to the enol, which is less stable due to the lower stability of the C=C-OH structure.
What happens when an alpha-beta unsaturated carbonyl compound is treated with NaOH?
-When treated with NaOH, the NaOH deprotonates the alpha-hydrogen, forming an enolate ion. The enolate ion is a strong nucleophile that can then attack an electrophilic carbonyl group, leading to further reactions such as aldol condensation or Michael addition, depending on the conditions and the structure of the compound.
How does the formation of the enolate ion facilitate nucleophilic attacks in these reactions?
-The enolate ion, formed by deprotonating the alpha-hydrogen, is highly nucleophilic due to the negative charge on the oxygen atom and the double bond on the carbon. This makes the enolate ion a strong nucleophile, capable of attacking electrophilic centers, such as the carbonyl carbon in other molecules, facilitating reactions like Michael addition or aldol condensation.
Why does the script suggest drawing mechanisms for reactions involving alpha-beta unsaturated carbonyl compounds?
-The script emphasizes the importance of drawing mechanisms because understanding the flow of electrons and the sequence of bond formations is crucial for predicting the correct products and understanding the underlying chemistry. Without proper mechanistic drawings, it becomes difficult to visualize the transition states and intermediates involved in these reactions.
What is the product formed when an alpha-beta unsaturated carbonyl compound undergoes Michael addition with NaOCH3?
-When an alpha-beta unsaturated carbonyl compound undergoes Michael addition with NaOCH3, the enolate ion formed by deprotonating the alpha-hydrogen attacks the beta carbon of the conjugated system, forming a new carbon-carbon bond. The final product is a beta-substituted carbonyl compound after protonation of the intermediate.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)