What is a Clinical Research Study?
Summary
TLDRThis video explains what clinical research studies, also known as clinical trials, are and why they matter. It highlights that these studies test whether a drug is safe and effective, using scientific data rather than opinions. The video emphasizes the importance of including diverse participants, explains study designs like placebo, double-blind, and randomized trials, and underscores patient safety and monitoring throughout. Participation is voluntary, requiring informed consent, and not all patients are eligible. Ultimately, the video encourages patients to discuss the benefits and risks with their doctors and make informed decisions about joining a clinical study.
Takeaways
- 🧪 Clinical study, clinical trial, and research study all refer to the same process of testing a drug or treatment for safety and effectiveness.
- 💊 Every medication, from common painkillers to advanced cancer treatments, undergoes clinical research before widespread use.
- 📊 Studies generate scientific data, not opinions, to answer questions about side effects, effectiveness, and treatment comparisons.
- 🌍 Diverse patient participation is crucial to understand how treatments work across different races, ancestries, genders, and other populations.
- 👩⚕️ Participants receive consistent care, tests, scans, and follow-ups, with safety procedures in place to monitor their well-being.
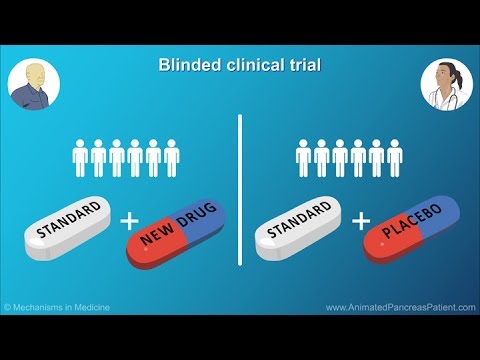
- ⚖️ Some studies are comparative, using standard treatments with or without the study drug, often under double-blind conditions.
- 🎲 Patients may be randomized into study groups, meaning they are assigned randomly to receive different treatments or a placebo.
- ✍️ Participation in a study is voluntary and requires informed consent, and patients can leave a study at any time.
- -
- 📋 Not every patient is eligible; enrollment is based on meeting specific criteria like age and illness severity.
- -
- ✅ Well-designed studies help determine if a drug is safe and effective, whether it works better than existing treatments, and guide future medical research.
Q & A
What is a clinical research study?
-A clinical research study, also called a clinical trial, is a scientific investigation designed to determine if a drug or treatment is safe and effective. It collects data on how the treatment works and its potential side effects.
Do the terms clinical study, clinical trial, and research study mean different things?
-No, all three terms refer to the same type of study aimed at evaluating the safety and effectiveness of a treatment or drug.
Why is it important to include patients of diverse races and backgrounds in studies?
-Diverse participation ensures researchers understand how a drug works across different populations, identifying any differences in effectiveness or side effects among groups with different races, ancestries, genders, or abilities.
What types of questions do clinical studies aim to answer?
-Studies aim to determine side effects, whether the treatment slows or stops the illness, how it compares to current treatments, and whether it can be safely combined with other drugs for the same condition.
What does a double-blind study mean?
-In a double-blind study, neither the patient nor the doctor knows whether the patient is receiving the study drug or a placebo, which helps remove bias from the results.
What is a placebo?
-A placebo is a substance that looks like the study drug but contains no active ingredients. It is used to compare the effect of the study drug against no treatment.
What does it mean for patients to be randomized in a study?
-Randomization means patients are assigned to different groups by chance, like flipping a coin, to ensure unbiased results and fair comparison between treatments.
Is participation in a clinical study mandatory?
-No, participation is completely voluntary. Patients must give informed consent before joining and can leave the study at any time.
What eligibility criteria must patients usually meet to join a study?
-Eligibility criteria often include being over 18 years old and having a specific illness severity. Each study has its own specific requirements that must be met for enrollment.
What benefits do patients receive while participating in a study?
-Patients receive personalized care, regular tests, scans, procedures, study visits, and continuous safety monitoring throughout the study.
How do clinical studies help doctors and researchers?
-Studies provide scientific data on whether a treatment works, its safety, how it compares to standard therapy, and guide future research and treatment decisions.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

An Introduction to Evidence Based Medicine

The Hawthorne Effect (Definition + Examples)

Scientist Explains: Common Myths & Misconceptions about Clinical Research
Understanding Clinical Trials

Introduction to Clinical Study Design: Introduction to Observational & Interventional Studies Part 2

ESTUDOS DA UTILIZAÇÃO DE MEDICAMENTOS | SÉRIE EDUCAÇÃO FARMACÊUTICA EM ONCOLOGIA
5.0 / 5 (0 votes)