Apa itu Atom? | Teori Perkembangan Atom - Kimia IPA
Summary
TLDRThis video provides a detailed exploration of atomic theory, beginning with the early ideas of Greek philosophers Leucippus and Democritus, who introduced the concept of atoms. It covers the progression of atomic models, from Dalton's solid ball model to Thomson's raisin bun theory, Rutherford's discovery of the atomic nucleus, Bohr's orbital model, and the modern quantum mechanical model. The video explains how each model contributed to our understanding of atomic structure and the behavior of electrons, leading to the current quantum mechanical model, which incorporates electron clouds and orbitals, offering the most accurate representation of atoms today.
Takeaways
- 😀 Greek philosophers Leucippus and Democritus first proposed that matter is made up of indivisible particles called atoms, around 450 BCE.
- 😀 An atom is the smallest part of a material that cannot be divided any further, as demonstrated by tearing a piece of paper into smaller and smaller pieces.
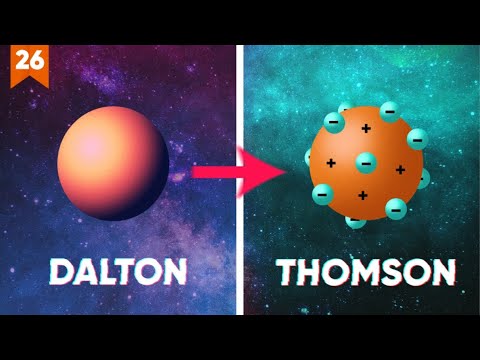
- 😀 John Dalton's atomic model described atoms as solid, indivisible balls, but his theory couldn't explain why atoms could conduct electricity.
- 😀 J.J. Thomson's atomic model, the 'raisin bun' model, proposed that atoms are a solid mass with positive charges and scattered electrons, but it couldn't explain the arrangement of charges.
- 😀 Ernest Rutherford's atomic model introduced the concept of a dense, positively charged nucleus surrounded by electrons, but it failed to explain why electrons don't spiral into the nucleus.
- 😀 Niels Bohr's atomic model, developed in 1913, introduced the concept of electron orbits or shells around a nucleus, but it did not align with the Heisenberg uncertainty principle.
- 😀 The quantum mechanical atomic model, the most modern theory, suggests that electrons exist in electron clouds and their positions cannot be precisely determined, only the probability of where they are likely to be found.
- 😀 In the quantum mechanical model, the electron cloud around the nucleus represents areas where electrons are likely to be, called orbitals.
- 😀 Orbitals form subshells, and subshells combine to form shells. There are four types of orbitals: s, p, d, and f.
- 😀 The quantum mechanical atomic model is currently the most accepted model, incorporating the principles of electron probability and orbital energy levels.
Q & A
Who were the first philosophers to propose the concept of atoms?
-The first philosophers to propose the concept of atoms were Leucippus and Democritus around 450 BC.
What is an atom as defined in the script?
-An atom is the smallest part of matter that cannot be divided further.
How can you physically demonstrate the concept of an atom?
-You can demonstrate the concept by tearing a paper into smaller and smaller pieces until it can't be divided anymore. The smallest part that cannot be divided is considered an atom.
What is Dalton’s atomic model, and how did it describe the atom?
-Dalton's atomic model described the atom as a solid, indivisible ball that was neutral, with atoms of different elements having different types.
What was the limitation of Dalton’s atomic model?
-Dalton’s atomic model could not explain how atoms conduct electricity, even though electricity involves moving electrons.
What was the key feature of Thomson’s atomic model?
-Thomson’s atomic model described the atom as a solid ball with a positive charge, with negative particles (electrons) scattered inside, resembling a 'raisin bun'.
What did Rutherford’s atomic model propose about the structure of the atom?
-Rutherford’s atomic model proposed that atoms have a central, positively charged nucleus, with electrons orbiting around it. The mass of the atom is concentrated in the nucleus, and the rest of the atom is mostly empty space.
What was the issue with Rutherford’s atomic model?
-Rutherford's atomic model could not explain why electrons, moving around the nucleus, do not lose energy and spiral into the nucleus, as predicted by physics.
What does the Bohr atomic model introduce that earlier models did not?
-The Bohr atomic model introduced the concept of specific orbits or shells where electrons exist, revolving around the nucleus, and it also provided a more defined structure for the atom.
What does the modern quantum mechanical atomic model describe about electron positions?
-The modern quantum mechanical atomic model suggests that the position of electrons cannot be precisely determined at the same time as their momentum. Instead, it describes the probability of finding an electron at a particular distance from the nucleus.
What are orbitals, and how are they organized in the quantum mechanical model?
-Orbitals in the quantum mechanical model are regions where electrons are likely to be found. These orbitals are grouped into subshells, and subshells are grouped into shells. There are four types of orbitals: s, p, d, and f.
How does the quantum mechanical model differ from earlier atomic models?
-The quantum mechanical model differs from earlier models by focusing on the probability of electron locations rather than fixed orbits, incorporating the uncertainty principle, and introducing the concept of orbitals and electron clouds.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

2.2.1 - Estudo do átomo - Hipótese atômica de Leucipo e Demócrito: Teoria Atomística

GCSE Chemistry | History of the Atom

Modelos de Dalton e Thomson [Módulo 02 - Aula 01]
O Modelo Atômico de Dalton x Thomson

Quarter 2_WEEK 1 - DAY 1 DALTON AND DEMOCRITUS | Science 8 MATATAG Curriculum

HISTORIA DEL ÁTOMO
5.0 / 5 (0 votes)