Changes Are Fun! Heating and Cooling | MightyOwl Science | 2nd Grade
Summary
TLDRIn this engaging video, Mighty Owl explores the science behind heating and cooling, showing how everyday activities like making s'mores or cooling a drink involve both reversible and non-reversible changes. The script covers a variety of examples, from heating marshmallows on a fire to cooling drinks with ice, highlighting how these processes affect the temperature and state of materials. The video delves into the concept of reversible changes, such as melting and freezing, as well as irreversible changes like cooking an egg or burning paper. With fun facts about temperature extremes, like Antarctica’s cold and Libya’s heat, the video brings science to life in a relatable way.
Takeaways
- 😀 Heating a marshmallow on a fire increases its temperature, which is an example of a heat source in action.
- 😀 Other common sources of heat include appliances like toasters, ovens, hair dryers, and even the sun.
- 😀 The sun's heat can be extreme, reaching temperatures as high as 136°F in places like Libya, the hottest recorded temperature on Earth.
- 😀 To cool down a bottle of water, you can place it in the fridge or add ice to reduce its temperature.
- 😀 Antarctica is an extremely cold place where temperatures can drop to -128.6°F, with dangerous wind chill effects that can freeze skin in 30 seconds.
- 😀 Reversible changes involve processes that can be undone, like melting ice cream or butter, and freezing it back to solid form.
- 😀 Heating water to 212°F turns it into steam, which can condense back into water when it cools.
- 😀 Non-reversible changes occur when heat causes permanent changes, such as cooking an egg or burning paper.
- 😀 When you heat paper, it burns and turns into ash, which cannot be changed back into paper.
- 😀 Clay, when heated in a kiln, turns into hard pottery, and once cooled, it cannot revert to its original clay form.
- 😀 Sand, when struck by lightning, turns into glass—a non-reversible change that cannot return to its sand state.
Q & A
What is the science behind making s'mores?
-Making s'mores involves heating a marshmallow over a fire, which increases its temperature. This process is a form of heating, where the fire, being a source of heat, causes the marshmallow to change, which is a scientific concept related to heat transfer.
What are some common sources of heat in daily life?
-Common sources of heat include appliances such as toasters, ovens, hair dryers, and irons. The sun also provides a significant source of heat, especially in places like deserts, where temperatures can rise to extreme levels.
What is the hottest temperature ever recorded on Earth?
-The hottest temperature ever recorded on Earth was 136°F (58°C) in Libya, which is extremely hot and can be dangerously dehydrating.
How can you cool down a bottle of water?
-You can cool down a bottle of water by placing it in the fridge or adding ice. Both methods reduce the temperature of the water.
What is the 30-30-30 rule in Antarctica?
-The 30-30-30 rule in Antarctica states that when the temperature is below 30°F, and the wind is blowing at 30 miles per hour, human skin can freeze in just 30 seconds due to the extreme cold and wind chill.
What is an example of a reversible change?
-A reversible change occurs when a substance changes form but can return to its original state. For example, melting ice cream on a hot day can be refrozen to return to its solid form.
Can you think of other examples of reversible changes?
-Other examples of reversible changes include melting butter and freezing water into ice. Both of these changes can be undone by either heating or cooling the substance.
What is a non-reversible change?
-A non-reversible change is one where the substance cannot return to its original form. For example, cooking an egg causes it to solidify, and it cannot be turned back into its raw state.
What happens to paper when it is heated?
-When paper is heated, it burns and turns into ash. This is an example of a non-reversible change, as once paper is burned, it cannot return to its original form.
How is glass formed from sand?
-When sand is heated to extremely high temperatures, such as from a lightning strike, it melts and becomes a liquid. When it cools down, it forms glass, which is a non-reversible change because it cannot be turned back into sand.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Hukum Termodinamika, Bagian 6: Hukum Kedua

Le Chatelier's principle

The Heating and Cooling Curve of a Substance

What Are Reversible Reactions? | Reactions | Chemistry | FuseSchool
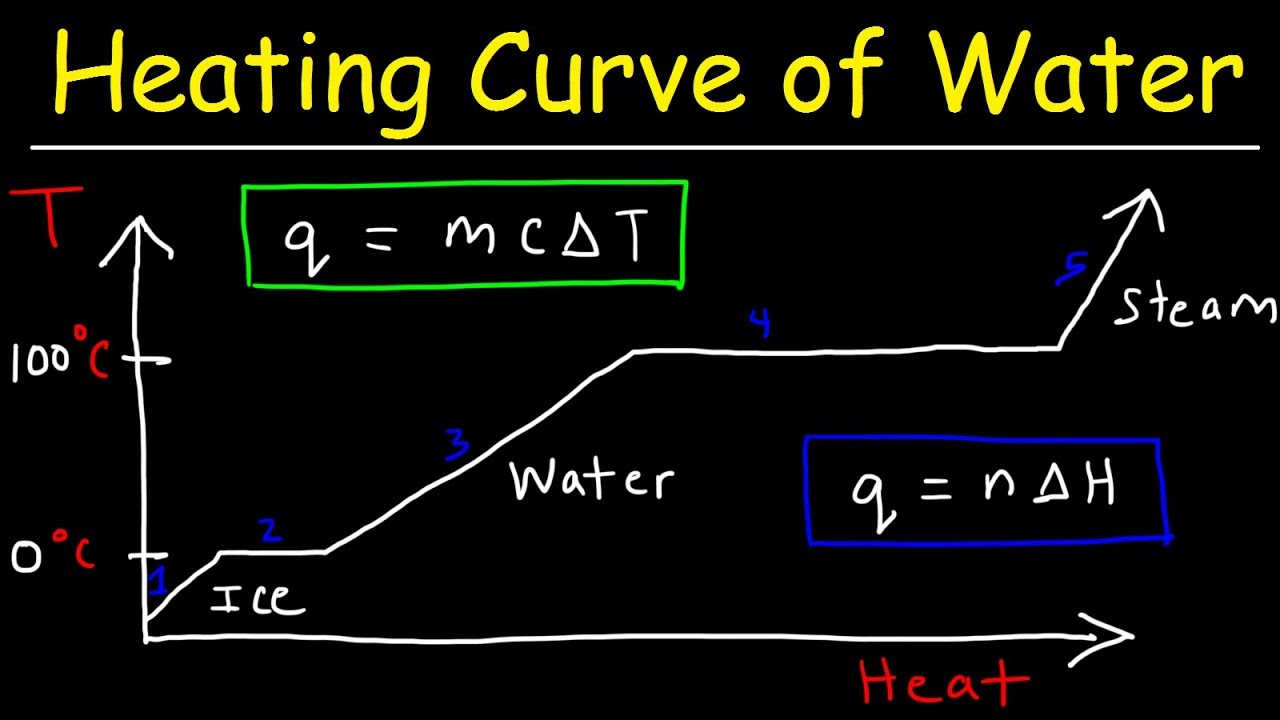
Heating Curve and Cooling Curve of Water - Enthalpy of Fusion & Vaporization

Cooling curve vs Heating curve Grade 10 Chemistry
5.0 / 5 (0 votes)