How to Find the Density of a Solid or Liquid
Summary
TLDRIn this educational video, we explore how to find the mass, volume, and density of different objects. Starting with simple shapes, like a wooden and a metal block, we calculate their densities using mass balance and volume formulas. For irregular objects, like a cylindrical item, we demonstrate using water displacement to measure volume. The video also covers techniques for determining the mass and density of liquids, like water and oil, using graduated cylinders. Through practical demonstrations, the video helps viewers understand the concepts of mass, volume, and density in everyday objects.
Takeaways
- 😀 The lesson involves calculating the mass, volume, and density of various objects to understand basic physical principles.
- 😀 The first objects studied are two blocks: one wooden and one metal, using a mass balance to determine their masses.
- 😀 The mass of the wooden block is 36.4 grams and the metal block is 467.7 grams.
- 😀 Volume of each block is determined by measuring its length, width, and height, resulting in a volume of 60 cm³ for both blocks.
- 😀 Density is calculated using the formula: density = mass ÷ volume. For the wood, the density is 0.6 g/cm³, and for the metal, it is 7.8 g/cm³.
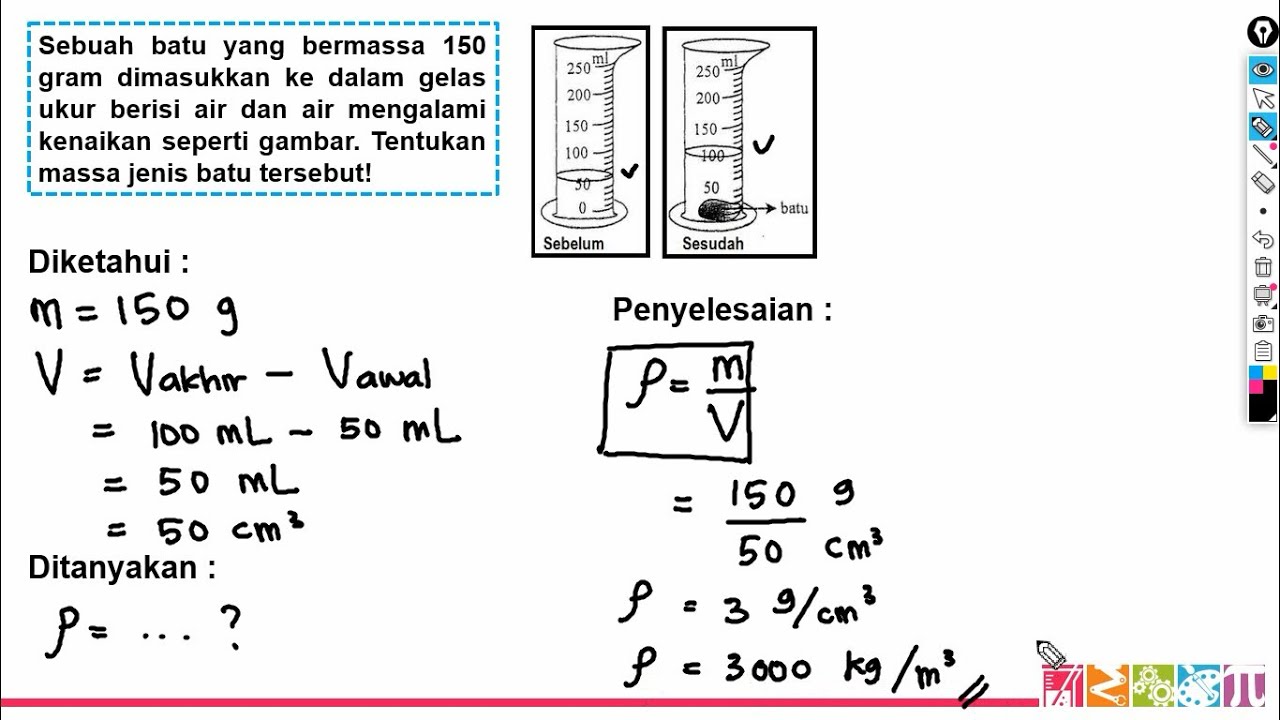
- 😀 When dealing with irregularly shaped objects, an overflow can method is used to measure volume by the amount of displaced water.
- 😀 The mass of an irregular object (99.3 g) is measured, and its volume is determined to be 11 cm³ by subtracting the displaced water volume.
- 😀 The density of the irregular object is calculated as 9 g/cm³ by dividing the mass (99.3 g) by the volume (11 cm³).
- 😀 For liquids, mass is not directly measurable with a simple balance, so a graduated cylinder is used to calculate mass by subtracting the mass of the empty cylinder.
- 😀 The density of water is calculated as 1.0 g/cm³, while the density of oil is found to be 0.9 g/cm³, meaning oil is less dense than water and will float on top.
- 😀 The demonstration highlights key scientific principles like mass, volume, density, and the use of water displacement to measure the volume of irregular objects.
Q & A
What is the purpose of the video?
-The purpose of the video is to demonstrate how to calculate the density of different objects by measuring their mass and volume, using both regular and irregular-shaped objects.
How is the density of the wooden block calculated?
-The density of the wooden block is calculated by dividing its mass (36.4 g) by its volume (60 cm³), resulting in a density of approximately 0.6 g/cm³.
What formula is used to calculate the density of an object?
-The formula used to calculate the density of an object is: Density = Mass / Volume.
How is the volume of the rectangular objects determined?
-The volume of the rectangular objects is determined by measuring their length, width, and height, and then multiplying these values together. For both blocks, the volume is 60 cm³.
What was the density of the metal block?
-The density of the metal block is calculated by dividing its mass (467.7 g) by its volume (60 cm³), resulting in a density of approximately 7.8 g/cm³.
How do you calculate the volume of an irregular object like the cylindrical one shown in the video?
-To calculate the volume of an irregular object, water displacement is used. The object is submerged in water, and the volume displaced by the object is measured, which is equal to the object's volume.
What method is used to find the volume of the irregular cylindrical object?
-The method used to find the volume of the irregular cylindrical object is water displacement. The initial water level is measured, and after the object is submerged, the new water level is recorded. The difference gives the volume.
What is the density of the irregular cylindrical object in the video?
-The density of the irregular cylindrical object is found by dividing its mass (99.3 g) by its volume (11 cm³), resulting in a density of approximately 9 g/cm³.
How is the mass of a liquid measured in the video?
-The mass of the liquid is measured by first recording the mass of the empty measuring cylinder. Then, the liquid is added, and the final mass is measured. The mass of the liquid is the difference between the final mass and the initial mass.
What is the density of oil compared to water?
-The density of the oil is calculated to be approximately 0.9 g/cm³, which is less than the density of water (1.0 g/cm³). This explains why oil floats on top of water.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)