Why is the carbon content in steel so important?
Summary
TLDRThis video delves into the various forms of steel and how their microstructures, influenced by carbon content, determine their mechanical properties. It explains the differences between low, medium, and high carbon steels, as well as cast irons, highlighting their uses in everyday items, tools, and industrial applications. The role of phase diagrams in understanding steel's equilibrium microstructure is emphasized, showcasing key phases like ferrite, austenite, and cementite. The video also explores the effects of eutectoid, hypo-eutectoid, and hyper-eutectoid compositions on steel's hardness and ductility, providing a comprehensive overview of the importance of carbon content in steel selection.
Takeaways
- 😀 Steels are alloys of iron and carbon, widely used in modern engineering due to their varied properties based on carbon content.
- 😀 Low carbon steels (0.04-0.3% carbon) are ductile, easy to machine and weld, and commonly used in applications like car body panels and re-bar reinforcements.
- 😀 Medium carbon steels (0.3-0.7% carbon) have higher hardness and are used in applications such as gears and cutting tools.
- 😀 High carbon steels (0.7-1.7% carbon) are harder, abrasion-resistant, and used in railway tracks but are more brittle and difficult to weld.
- 😀 Cast irons, with carbon content above 1.7%, are very hard and brittle, making them suitable for kitchen pots and pans.
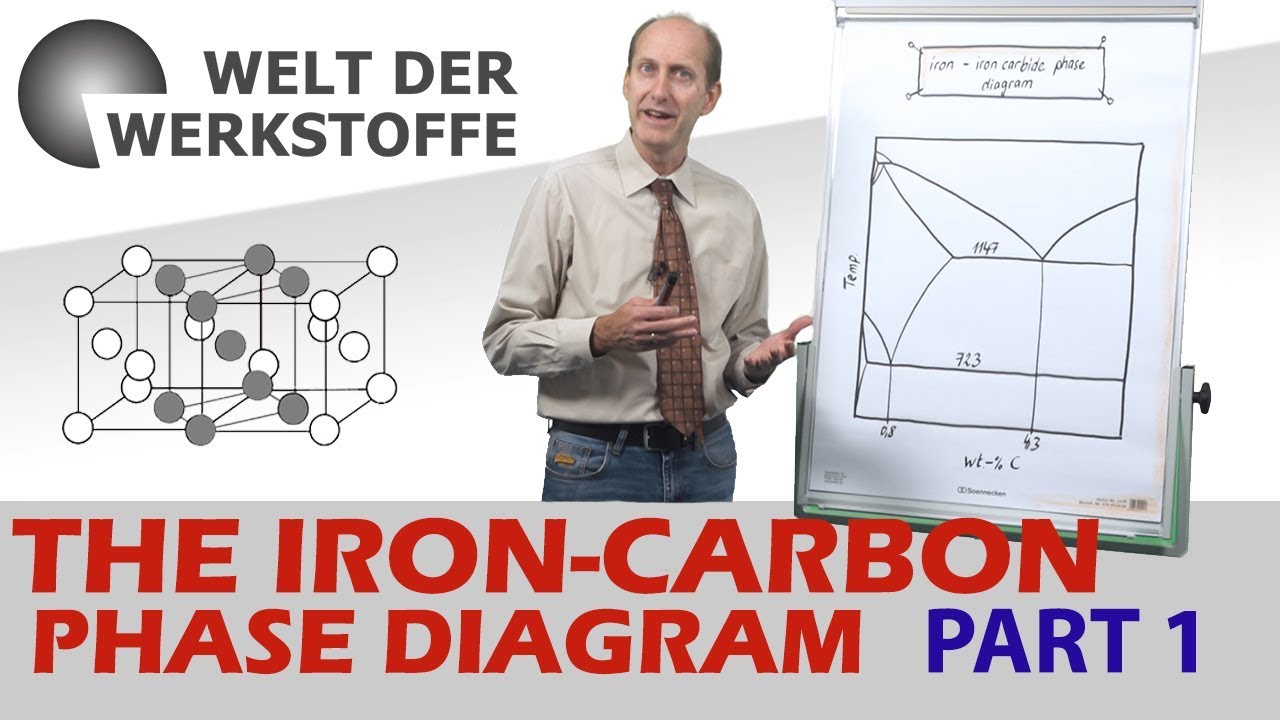
- 😀 Understanding the phases in steel is crucial for controlling mechanical properties, and phase diagrams help predict microstructure formation at different temperatures and compositions.
- 😀 The iron-carbon phase diagram is key in determining the phase transitions and equilibrium microstructure of steel based on its carbon content.
- 😀 The eutectoid composition (0.76% carbon) results in pearlite, a 2-phase microstructure consisting of alternating layers of ferrite (soft) and cementite (hard).
- 😀 A hypo-eutectoid steel (less than 0.76% carbon) forms pearlite held together with soft pro-eutectoid ferrite.
- 😀 A hyper-eutectoid steel (more than 0.76% carbon) forms pearlite with harder pro-eutectoid cementite, increasing hardness.
- 😀 The phase diagram shows important points such as the eutectic point (4.3% carbon) and eutectoid point (0.76% carbon), both influencing the steel's mechanical properties.
Q & A
What are the different types of steel based on carbon content?
-Steel is classified into low, medium, and high carbon steels, depending on the carbon content. Low carbon steels contain 0.04-0.3% carbon, medium carbon steels have 0.3-0.7%, and high carbon steels have 0.7-1.7%. Cast irons, which are not technically steel, have carbon content above 1.7%.
How does increasing the carbon content in steel affect its properties?
-As the carbon content increases, the hardness of steel increases, making it more wear-resistant but also more brittle. This can make the steel ideal for applications like cutting tools or railway lines, but also prone to fracture at higher carbon levels.
What is the role of phases in determining the properties of steel?
-Phases in steel, such as ferrite, austenite, and cementite, play a key role in determining its mechanical properties. For example, ferrite is softer and more ductile, while cementite is hard and brittle. The proportions and interactions of these phases affect the steel's strength, hardness, and ductility.
What is the eutectoid composition in steel, and why is it important?
-The eutectoid composition in steel is 0.76% carbon, where the material forms a microstructure known as pearlite, consisting of alternating layers of soft ferrite and hard cementite. This composition is significant because it results in a balanced combination of strength and ductility.
What is the difference between hypo-eutectoid and hyper-eutectoid steel?
-In hypo-eutectoid steel (less than 0.76% carbon), pearlite grains are held together by soft ferrite, which results in a softer material. In hyper-eutectoid steel (more than 0.76% carbon), pearlite grains are held together by hard cementite, making the steel harder and more brittle.
What is the significance of cementite in steel?
-Cementite (Fe3C) is a hard and brittle phase in steel that significantly affects its mechanical properties. The amount of cementite in the alloy determines its hardness. More cementite results in a harder but more brittle material, while less cementite results in a softer and more ductile steel.
How does the iron-carbon phase diagram help in understanding steel properties?
-The iron-carbon phase diagram helps in visualizing how the microstructure of steel changes with temperature and carbon content. It shows how phases like ferrite, austenite, and cementite form and interact at different compositions and temperatures, allowing for better material selection for specific applications.
What is pearlite, and how does it form in steel?
-Pearlite is a two-phase microstructure in steel that forms at the eutectoid composition (0.76% carbon). It consists of alternating layers of soft ferrite and hard cementite. As steel cools from the austenite phase, pearlite forms at temperatures below 727°C.
What is the role of temperature in determining the microstructure of steel?
-Temperature plays a crucial role in determining which phases form in steel. At higher temperatures, austenite (a soft and non-magnetic phase) forms. As the material cools, it can transform into ferrite, cementite, or pearlite, depending on the carbon content and temperature.
What is the difference between austenite and ferrite in steel?
-Austenite is a phase of iron that forms at high temperatures (above 910°C), characterized by a face-centered cubic (FCC) structure. It is soft and non-magnetic. Ferrite, on the other hand, forms at lower temperatures (below 910°C) and has a body-centered cubic (BCC) structure, making it soft and magnetic.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)