(1 dari 5) Farmasi Fisika 2021 - Fenomena Antarmuka - Konsep & Pendahuluan
Summary
TLDRThis lecture delves into interfacial phenomena and surface tension, focusing on their significance in the pharmaceutical world. Key concepts such as cohesive and adhesive forces, surfactants, and their role in emulsions and suspensions are explained. The importance of surface tension in preventing objects from sinking in water, as well as how surfactants affect surface tension, is explored. Practical examples and measurement methods, including the force needed to stretch a soap film, are also highlighted, making this an essential topic for understanding various pharmaceutical applications.
Takeaways
- 😀 Surface tension is a key concept that defines the force on the surface of a liquid, preventing objects from sinking despite having a higher density than water.
- 😀 Cohesion forces are the attractive forces between molecules of the same type, while adhesion forces involve the interaction between different types of molecules, such as water and air.
- 😀 The interface phenomenon occurs when different phases meet, with surface tension being most prominent when a liquid interacts with air.
- 😀 Objects can float on water because the cohesive forces between water molecules create a surface layer that supports them.
- 😀 In interactions between liquids and solids (like in emulsions), interfacial tension plays a significant role, which differs from surface tension found with liquids and air.
- 😀 The concept of surface tension is essential in pharmacy, especially when considering the interactions in emulsions and suspensions.
- 😀 Surface tension can be measured through a balance experiment, where the force required to break the surface layer is recorded.
- 😀 When the force applied to the liquid’s surface exceeds its surface tension, the liquid will break or the object will sink.
- 😀 Surface tension is crucial for understanding various physical phenomena, including how liquids interact with each other and solid surfaces.
- 😀 In the pharmaceutical field, understanding surface tension and interfacial phenomena is vital for applications such as drug formulations and delivery systems.
- 😀 The discussion also touches on the relationship between cohesion and adhesion forces, explaining their role in the behavior of liquids at interfaces.
Q & A
What is the main topic discussed in this transcript?
-The main topic of the transcript is 'Interfacial Phenomena and Surface Tension,' focusing on surface tension, adsorption, and the role of surfactants in the context of physics and pharmacy.
Why do certain objects not sink in water even though their density is greater than that of water?
-Certain objects do not sink in water due to surface tension, which creates a thin 'layer' on the water's surface that resists the force of gravity, preventing these objects from sinking.
What is surface tension and how is it explained in the transcript?
-Surface tension is the force at the surface of a liquid that prevents the liquid from breaking apart. It is caused by cohesive forces between the molecules of the liquid, which are stronger at the surface, creating a 'skin' that resists external forces.
What is the difference between cohesive force and adhesive force?
-Cohesive force is the force between molecules of the same type, while adhesive force is the force between molecules of different types. In the case of water, cohesive forces are stronger than adhesive forces, causing water molecules to stick together and form surface tension.
How does the concept of surface tension apply to objects placed on water?
-When an object is placed on water, the surface tension acts to hold the object on the water's surface. If the object is light enough, the surface tension will prevent it from sinking. If the weight exceeds the surface tension, the object will sink.
What is the difference between surface tension and interfacial tension?
-Surface tension occurs at the interface between a liquid and air, while interfacial tension occurs at the interface between two immiscible liquids or solids. Surface tension is a specific type of interfacial tension, which is a broader term used for different phase interactions.
What are the types of interfaces where surface or interfacial tension occurs?
-Surface tension occurs at liquid-air interfaces, while interfacial tension can occur between different phases, such as liquid-liquid, liquid-solid, or solid-solid, when the phases do not mix.
How is surface tension measured in a laboratory setting?
-Surface tension can be measured by applying a force to a liquid's surface, such as using a wire or soap film. The force required to maintain the surface without breaking can be measured to determine the surface tension.
What is the role of surfactants in surface tension?
-Surfactants reduce surface tension by decreasing the cohesive forces between liquid molecules. They are used to modify surface properties in various applications, such as in emulsions and suspensions in the pharmaceutical field.
What are the phases discussed in relation to interfacial phenomena?
-The phases discussed include liquid-liquid, liquid-solid, and gas-solid interfaces, particularly emphasizing liquid and solid interactions in emulsions and suspensions.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Introduction to sprays and their applications

Surface tension | States of matter and intermolecular forces | Chemistry | Khan Academy
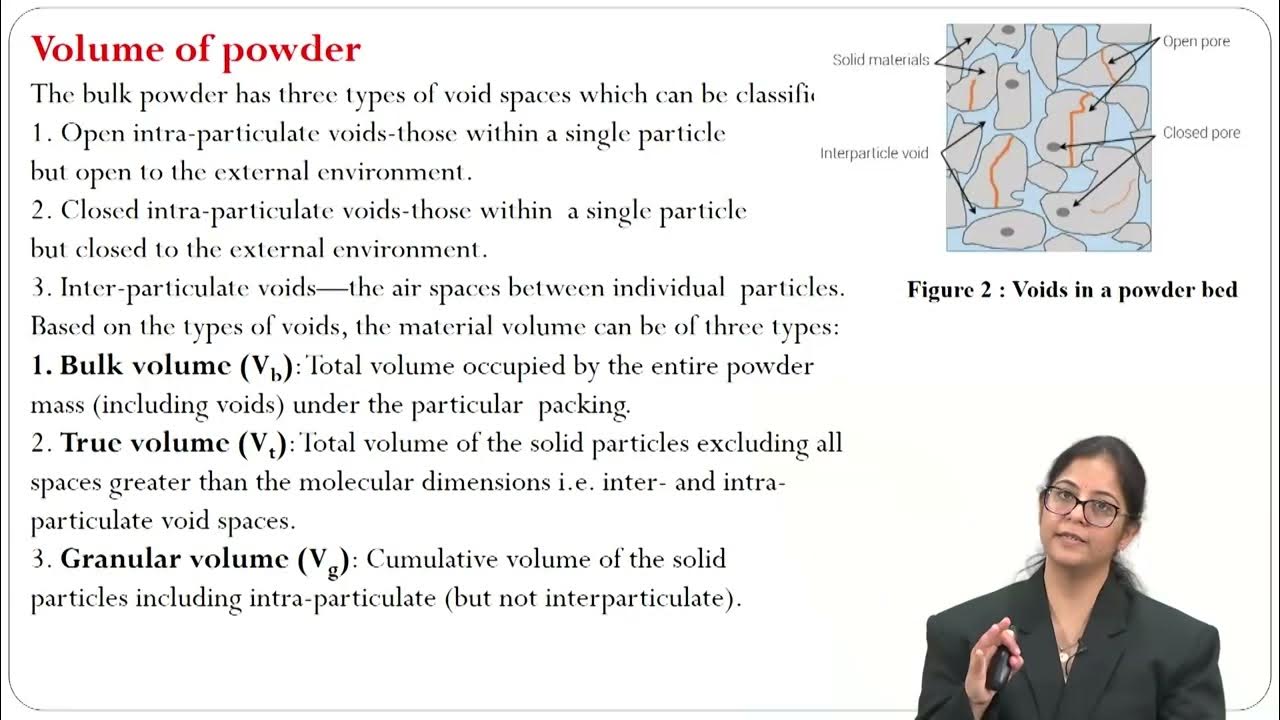
Fundamentals of Pharmaceutical Processes Compaction

FISIKA Kelas 11 - Tegangan Permukaan | GIA Academy
Melt pool dynamics

Tegangan Permukaan (Konsep, Rumus, Aplikasi, dan Contoh Soal)
5.0 / 5 (0 votes)