(16) IR Spectroscopy | Introduction to Infrared (IR) Spectroscopy | Instrumental Method of Analysis
Summary
TLDRThis video provides an in-depth introduction to IR (Infrared) Spectroscopy, focusing on its basic principles and applications. It covers the use of infrared radiation to analyze molecular structures through vibrational transitions. The video explains the electromagnetic spectrum, the division of the IR region into fingerprint and functional group regions, and the key components of the IR spectrum. Additionally, it highlights the three types of IR regions: near, mid, and far IR, and discusses the theory behind molecular vibrations. The session concludes with insights on how IR absorption occurs, providing a foundational understanding of IR spectroscopy.
Takeaways
- 😀 IR spectroscopy is the study of molecular vibrations using infrared light from the electromagnetic spectrum.
- 😀 It is also known as vibrational spectroscopy because it focuses on the vibration and rotation of molecules.
- 😀 The infrared region of the electromagnetic spectrum is between visible and microwave light, typically spanning 400 to 4000 cm⁻¹.
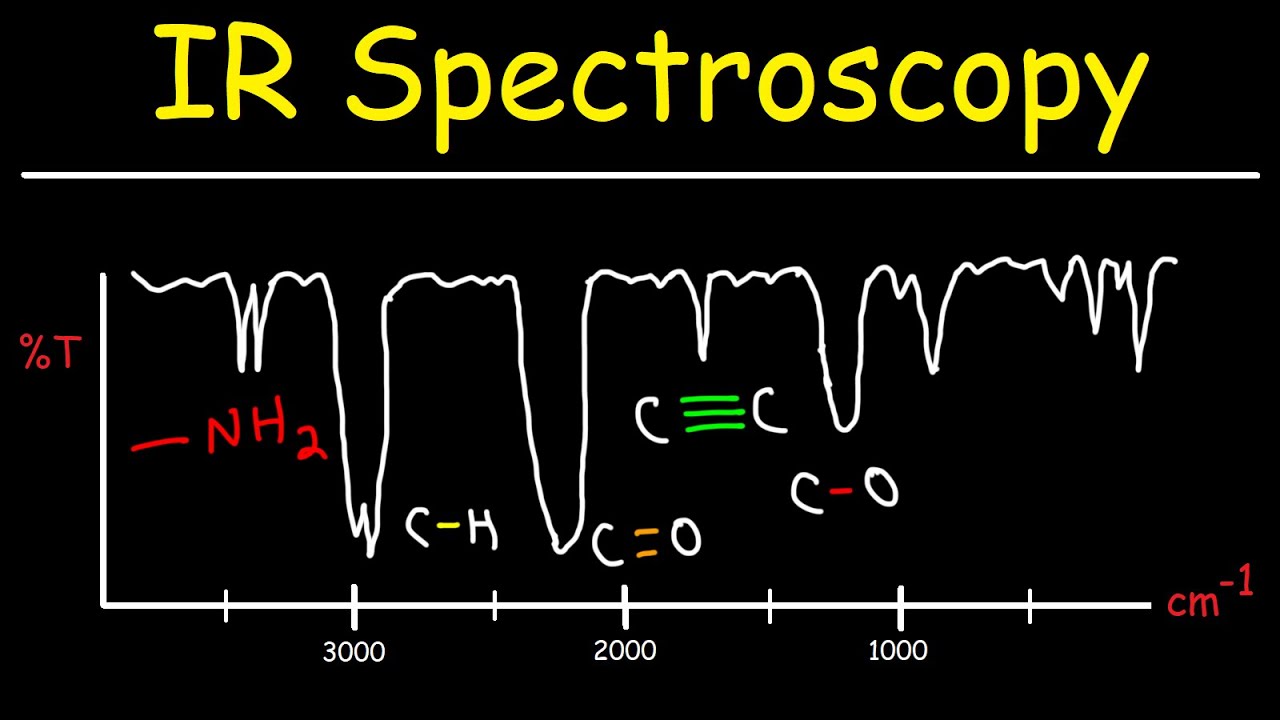
- 😀 The IR spectrum is divided into two main regions: the fingerprint region (400 to 1500 cm⁻¹) and the functional group region (1500 to 4000 cm⁻¹).
- 😀 The fingerprint region helps in identifying specific compounds, as each molecule has a unique IR fingerprint.
- 😀 The functional group region helps identify specific functional groups, such as -OH, -NH2, and -COOH, in a molecule.
- 😀 IR radiation is divided into three parts: near IR, mid IR, and far IR, each with different wavelengths and frequencies.
- 😀 Near IR typically has a wavelength of 0.8 to 2.5 µm and a wave number range of 12500 to 4000 cm⁻¹, while mid IR is 2.5 to 50 µm (4000 to 200 cm⁻¹).
- 😀 IR spectroscopy works on the principle that molecules vibrate like a spring and ball system, with atoms connected by chemical bonds.
- 😀 Vibrational transitions occur when the applied IR frequency matches the natural vibration frequency of the molecule's bonds, causing absorption and resulting in a peak in the spectrum.
Q & A
What is IR spectroscopy?
-IR spectroscopy, or Infrared Spectroscopy, is a technique that uses infrared light to study the structure of molecules. It involves the absorption of infrared radiation by molecules, leading to vibrational transitions, which helps in understanding molecular properties.
Why is IR spectroscopy also called vibrational spectroscopy?
-IR spectroscopy is also called vibrational spectroscopy because the absorption of infrared radiation causes vibrational transitions in molecules. These vibrations and rotations help analyze the molecular structure.
Where is the infrared region located in the electromagnetic spectrum?
-The infrared region is located between the visible and microwave regions of the electromagnetic spectrum. It spans wavelengths from approximately 0.8 micrometers to 1 millimeter.
What are the two main regions of the infrared spectrum?
-The infrared spectrum is divided into two main regions: the fingerprint region (400–1500 cm^-1) and the functional group region (1500–4000 cm^-1).
What is the fingerprint region in IR spectroscopy?
-The fingerprint region (400–1500 cm^-1) of the IR spectrum contains unique absorption bands that are specific to individual molecules, similar to how human fingerprints are unique.
What is the functional group region in IR spectroscopy?
-The functional group region (1500–4000 cm^-1) in IR spectroscopy shows characteristic absorption bands associated with different functional groups in molecules, like OH, NH, and CH groups.
How is the IR spectrum divided into near, mid, and far IR regions?
-The IR spectrum is divided into three parts: Near IR (12500–4000 cm^-1), Mid IR (4000–200 cm^-1), and Far IR (200–10 cm^-1). Near IR is used for overtone transitions, Mid IR for vibrations and rotations, and Far IR for rotational transitions.
What are the basic characteristics of molecules in IR spectroscopy?
-In IR spectroscopy, molecules are modeled as atoms connected by chemical bonds, which behave like springs. The vibrations between atoms in these bonds are similar to the stretching and compressing of a spring, which can be detected using IR radiation.
What happens when a molecule absorbs IR radiation?
-When a molecule absorbs IR radiation, its atoms vibrate more intensely. This occurs when the frequency of the applied IR radiation matches the molecule's natural vibrational frequency, leading to a transition from the ground state to an excited state.
What are the two key conditions for IR absorption to occur?
-For IR absorption to occur, two conditions must be met: 1) The frequency of the applied IR radiation must match the molecule's natural vibrational frequency. 2) There must be a change in the dipole moment of the molecule during vibration, creating a shift in the positive and negative charges on the atoms.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

IR Spectroscopy
IR Spectroscopy - Basic Introduction

Special Imaging Techniques 1: Basics

Infrared Spectroscopy: Key Features of Organic Functional Groups // HSC Chemistry
Fourier Transform IR spectroscopy (FTIR) - How it works?
UAD - Kuliah Online 1475530 Karakterisasi Material Lanjut (Lecture 2a - part 1)
5.0 / 5 (0 votes)