GCSE Chemistry Revision "Elements, Compounds and Mixtures"
Summary
TLDRIn this educational video, the concept of elements, compounds, mixtures, and molecules is explained clearly. Viewers learn that elements are pure substances made of identical atoms, compounds are made of different elements combined in fixed proportions, and mixtures contain different elements or compounds not chemically combined. The video also covers the distinction between molecules and compounds, where molecules can consist of the same or different elements. Practical examples like magnesium, sulfur, and magnesium sulfide illustrate these concepts. The video emphasizes the importance of these ideas in chemistry and introduces physical separation techniques for mixtures.
Takeaways
- 😀 Elements are pure substances made of only one type of atom, and there are around 100 different elements, each represented by a symbol in the periodic table.
- 😀 The definition of an element is that all the atoms in it are the same, as shown with magnesium and sulfur examples.
- 😀 Every element has a unique symbol, which starts with a capital letter (e.g., Magnesium = Mg, Sulfur = S).
- 😀 A compound is a substance formed by two or more different elements chemically combined in fixed proportions.
- 😀 In a compound like magnesium sulfide, the proportion of atoms of each element is fixed, such as one magnesium atom for every sulfur atom.
- 😀 Compounds often have different properties from the elements they are made from (e.g., magnesium sulfide is white crystals, while magnesium is a shiny metal and sulfur is a yellow solid).
- 😀 To separate a compound back into its elements, a chemical reaction is required, which is not always easy to do.
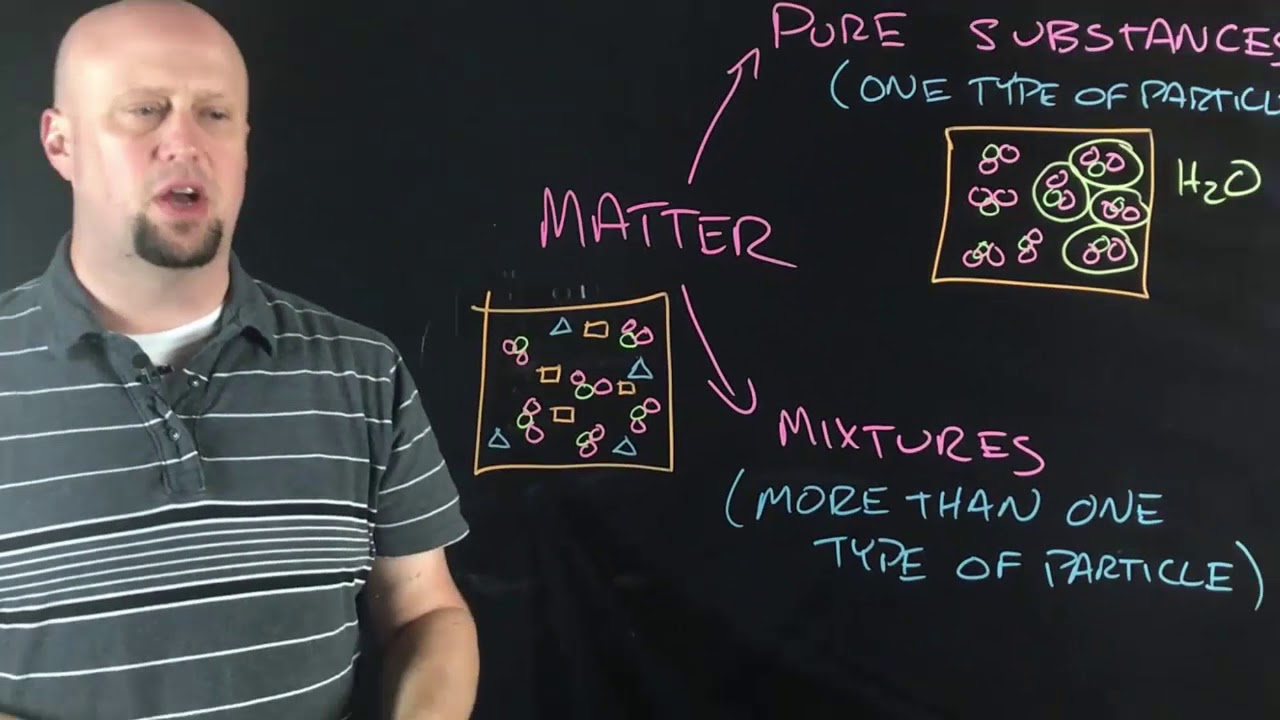
- 😀 A mixture contains different elements or compounds that are not chemically combined, and physical separation techniques can be used to separate them (e.g., filtration, distillation, crystallization, chromatography).
- 😀 A molecule is a group of atoms chemically combined, and it can consist of the same or different elements.
- 😀 Some molecules, like methane, water, and ammonia, are compounds, while others, like chlorine and oxygen, are elements in molecule form, not compounds.
- 😀 Understanding the difference between elements, compounds, mixtures, and molecules is crucial for chemistry, and the definitions should be learned for exams.
Q & A
What is an element?
-An element is a substance made up of atoms that are all the same. Each element is represented by a unique symbol, such as 'Mg' for magnesium or 'S' for sulfur.
How are elements represented in the periodic table?
-Elements are represented by symbols in the periodic table, with each symbol consisting of a capital letter, sometimes followed by a lowercase letter, like 'Mg' for magnesium or 'S' for sulfur.
What makes a compound different from an element?
-A compound is made when two or more different elements chemically combine in fixed proportions, whereas an element consists of only one type of atom.
What are the key properties of compounds?
-Compounds usually have different properties from the elements that form them. For example, magnesium is a shiny metal, sulfur is a yellow solid, but magnesium sulfide forms white crystals.
What does 'fixed proportions' mean in the context of compounds?
-'Fixed proportions' means that the elements in a compound combine in a specific ratio. For instance, in magnesium sulfide (MgS), there is a fixed 1:1 ratio of magnesium to sulfur atoms.
Can a compound be separated back into its elements easily?
-No, a compound cannot be separated back into its elements easily. It requires a chemical reaction, which is often difficult to perform.
What is a mixture, and how is it different from a compound?
-A mixture is a combination of different elements or compounds that are not chemically combined. Unlike compounds, mixtures can be separated by physical means, such as filtration or distillation.
What physical separation methods can be used to separate mixtures?
-Physical separation techniques such as filtration, distillation, crystallization, and chromatography can be used to separate mixtures.
What is the difference between a molecule and a compound?
-A molecule consists of atoms chemically combined, and it can contain the same or different types of elements. A compound is a specific type of molecule that contains different elements chemically bonded in fixed proportions.
Are oxygen (O₂) and chlorine (Cl₂) considered compounds? Why or why not?
-No, oxygen (O₂) and chlorine (Cl₂) are not compounds. They are molecules of the same element, so they are considered molecules but not compounds because compounds require different elements chemically bonded together.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

GCSE Science Revision Chemistry "Elements, Compounds and Mixtures"

UNSUR, SENYAWA, DAN CAMPURAN - IPA KELAS 8 SMP KURIKULUM MERDEKA
Classification pt 1 Pure Substances

Classification of matter | Structure and properties of matter | High school chemistry | Khan Academy

From Elements to Compounds and Mixtures!

GCSE Chemistry - Differences Between Compounds, Molecules & Mixtures #3
5.0 / 5 (0 votes)