Belajar Kimia : Larutan Penyangga/Buffer Part 1
Summary
TLDRThis script provides an in-depth explanation of buffer solutions, their importance in maintaining pH stability in biological systems like blood, and how they work. It covers the types of buffer solutions (acidic and basic) and the processes involved in preparing them through direct and indirect reactions. The transcript also emphasizes the need for specific acid-base combinations for buffer formation, giving examples such as acetic acid and ammonium hydroxide. Additionally, it includes practical examples of buffer solution problems and how to identify suitable combinations for creating effective buffers in different pH ranges.
Takeaways
- 😀 Buffer solutions (larutan penyangga) maintain a stable pH by resisting changes when acids or bases are added.
- 😀 Blood is an example of a buffer solution that helps maintain a pH range of 6.1 to 7.3, ensuring optimal oxygen binding by hemoglobin.
- 😀 Buffer solutions are made from a weak acid and its conjugate base or a weak base and its conjugate acid.
- 😀 There are two types of buffer solutions: acidic buffers (pH below 7) and basic buffers (pH above 7).
- 😀 Buffer solutions in the blood help maintain a stable pH, even when consuming acidic or basic foods.
- 😀 The blood buffer system includes carbonic acid (H2CO3) and bicarbonate (HCO3-), which help regulate pH levels.
- 😀 Intracellular buffer solutions consist of H2PO4- and HPO4^2-, helping regulate the pH inside cells.
- 😀 Acidic buffers can be created by directly mixing a weak acid with its conjugate base, or indirectly by reacting a weak acid with a strong base.
- 😀 Basic buffers are created by mixing a weak base with its conjugate acid, either directly or through an indirect reaction with a strong acid.
- 😀 For buffer solutions to work, the concentration of the weak acid or weak base must be higher than that of the strong acid or base added.
- 😀 In buffer preparation, the molar concentrations of acid and base play a critical role in determining the pH that the buffer can resist.
Q & A
What is a buffer solution, and what is its main function?
-A buffer solution is a solution that resists changes in pH when an acid or base is added. Its main function is to maintain a stable pH, preventing significant fluctuations in pH when small amounts of acids or bases are introduced.
How does a buffer solution maintain pH in the human blood?
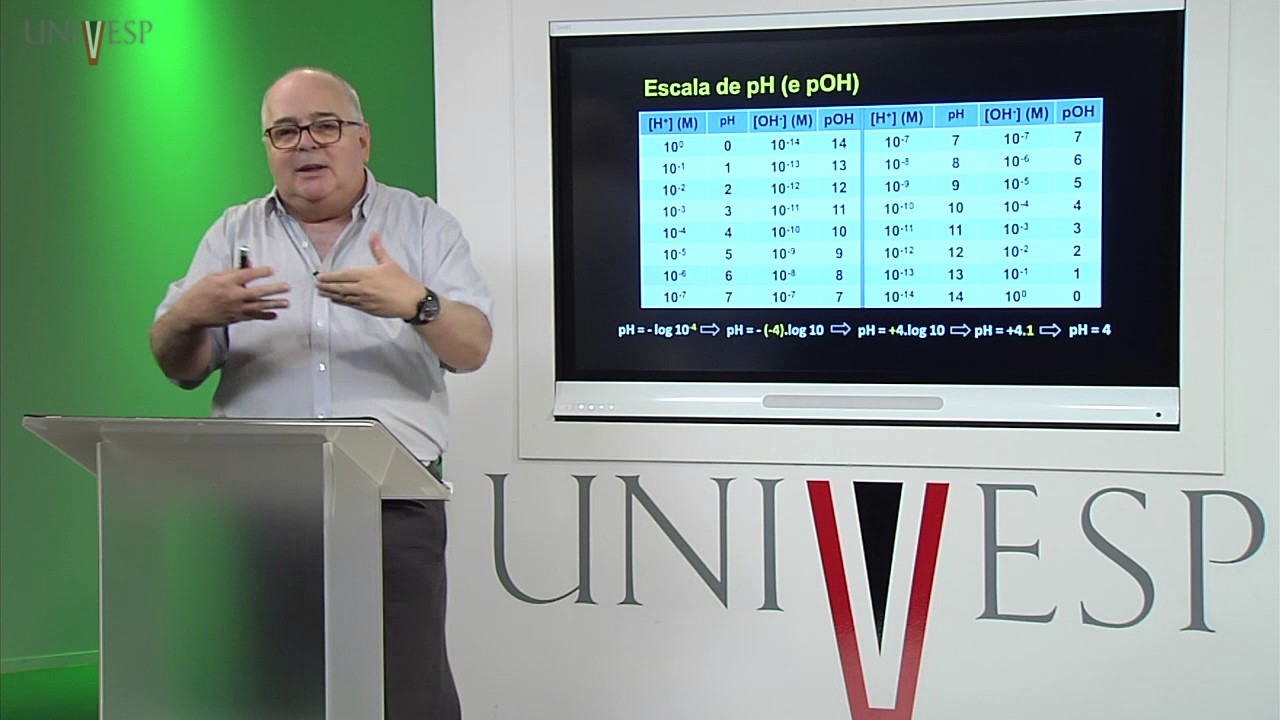
-In human blood, buffer solutions, such as the carbonic acid (H2CO3) and bicarbonate (HCO3-) system, maintain pH between 6.1 and 7.3. This range is optimal for oxygen binding by hemoglobin. If the pH deviates from this range, blood's ability to carry oxygen becomes impaired.
What are the two types of buffer solutions?
-The two types of buffer solutions are acidic buffers, which maintain a pH below 7, and basic buffers, which maintain a pH above 7.
How can an acidic buffer solution be created?
-An acidic buffer solution can be created by mixing a weak acid with its conjugate base. For example, acetic acid (CH3COOH) can be combined with its conjugate base, acetate (CH3COO-), to form an acidic buffer.
What is the difference between a direct and indirect method for preparing buffer solutions?
-In the direct method, the weak acid and its conjugate base are mixed directly to form a buffer. In the indirect method, the weak acid first reacts with a strong base to form a salt, which then reacts with more acid to establish the buffer solution.
What is the role of conjugate bases in buffer solutions?
-Conjugate bases in buffer solutions are responsible for accepting H+ ions when an acid is added, preventing a significant drop in pH. This is crucial for maintaining pH stability.
What makes blood an effective buffer in the human body?
-Blood contains a buffer system, mainly based on carbonic acid (H2CO3) and bicarbonate (HCO3-), which helps maintain the pH within the optimal range of 6.1 to 7.3. This system effectively stabilizes the blood pH despite the intake of acidic or basic foods.
What is the consequence if the blood pH goes beyond the optimal range of 6.1 to 7.3?
-If the blood pH exceeds the range of 6.1 to 7.3, the function of hemoglobin in oxygen binding becomes impaired, leading to potential health issues such as respiratory or metabolic disturbances.
What is an example of a base conjugate, and how is it related to buffer solutions?
-An example of a base conjugate is bicarbonate (HCO3-) formed from the dissociation of carbonic acid (H2CO3). It helps neutralize excess H+ ions in the blood, maintaining pH stability.
What is the importance of understanding weak acids and their conjugate bases when preparing buffer solutions?
-Understanding weak acids and their conjugate bases is essential because the buffer solution’s capacity to resist pH changes depends on the relative concentrations of the weak acid and its conjugate base. Knowing which acids and bases are weak or strong helps in selecting the appropriate combination for creating effective buffers.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

SOLUÇÃO TAMPÃO | EQUILÍBRIO QUÍMICO | Aula 26
Bioquímica - Aula 03 - Alguns conceitos químicos importantes - 2

Larutan Penyangga • Part 1: Sifat, Komponen & Peran Larutan Buffer / Penyangga

SIFAT SIFAT DAN KEGUNAAN LARUTAN PENYANGGA

Buffers (A-level IB Chemistry)

KIMIA Kelas 11 - Larutan Penyangga | GIA Academy
5.0 / 5 (0 votes)